No significant increase in Guillain-Barré syndrome after COVID-19 vaccination in adults: A vaccine adverse event reporting system study
- PMID: 36055875
- PMCID: PMC9393181
- DOI: 10.1016/j.vaccine.2022.08.038
No significant increase in Guillain-Barré syndrome after COVID-19 vaccination in adults: A vaccine adverse event reporting system study
Abstract
Objective: To investigate the association between Guillain-Barré syndrome (GBS) and COVID-19 vaccination.
Background: On July 13, 2021, the US Food and Drug Administration (FDA) released a new warning that Johnson & Johnson COVID-19 vaccine could increase the risk of developing GBS.
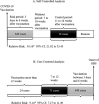
Methods: The reporting rate of adult GBS after COVID-19 vaccination, ascertained with Brighton criteria, was compared with the reporting rate after other vaccinations during the same time period, and also compared with the reporting rate during control periods. Statistical methods such as proportion tests, and Pearson's chi-squared test were utilized to identify significant relationships. Self-controlled and case centered analyses were conducted. A machine learning model was utilized to identify the factors associated with a worse outcome defined as emergency room (ER) or doctor visits, hospitalizations, and deaths.
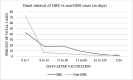
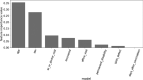
Results: The reporting rate of GBS after COVID-19 vaccination was significantly higher than after influenza and other vaccinations (49.7, 0.19, 0.16 per 10 million, p < 0.0001). However, the reporting rate was within the incidence range of GBS in the general population. Using self-controlled and case centered analyses, there was a significant difference in the reporting rate of GBS after COVID-19 vaccination between the risk period and control period (p < 0.0001). There was an estimated 0.7-1.7 per million excess reports of GBS within 6 weeks of COVID-19 vaccination. Machine learning model demonstrated that female gender and age between 18 and 44 are associated with worse outcome. No association was found between the onset interval of GBS and its prognosis.
Conclusions: Although the reporting rate of GBS after COVID-19 vaccination was not statistically different than that of the general population, the increased reporting of GBS within the first 6 weeks after COVID-19 vaccination, more so than with other vaccinations, suggests that some cases of GBS are temporally associated with COVID-19 vaccination. However, there is a reduction in the reporting rate of GBS after other vaccines, compared to reporting rates pre-COVID-19, highlighting limitations inherent in any passive surveillance system. These findings warrant continuous analysis of GBS after COVID-19 vaccination. Further improvement of the machine learning model is needed for clinical use.
Keywords: COVID-19 vaccination; Guillan Barre syndrome; Machine learning; SARS-CoV-2; Vaccine adverse events.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021.JAMA. 2021 Oct 26;326(16):1606-1613. doi: 10.1001/jama.2021.16496. JAMA. 2021. PMID: 34617967 Free PMC article.
-
Exploring the adverse events of Oxford-AstraZeneca, Pfizer-BioNTech, Moderna, and Johnson and Johnson COVID-19 vaccination on Guillain-Barré Syndrome.Sci Rep. 2024 Aug 13;14(1):18767. doi: 10.1038/s41598-024-66999-7. Sci Rep. 2024. PMID: 39138276 Free PMC article.
-
Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States.JAMA Netw Open. 2023 Feb 1;6(2):e2253845. doi: 10.1001/jamanetworkopen.2022.53845. JAMA Netw Open. 2023. PMID: 36723942 Free PMC article.
-
Guillain-Barré syndrome and COVID-19 vaccination: a systematic review and meta-analysis.J Neurol. 2024 Mar;271(3):1063-1071. doi: 10.1007/s00415-024-12186-7. Epub 2024 Jan 17. J Neurol. 2024. PMID: 38233678 Free PMC article.
-
Effects of COVID-19 vaccine type on Guillain-Barré syndrome: Two cases and a literature review.Hum Vaccin Immunother. 2023 Dec 31;19(1):2171231. doi: 10.1080/21645515.2023.2171231. Epub 2023 Feb 22. Hum Vaccin Immunother. 2023. PMID: 36919452 Free PMC article. Review.
Cited by
-
Diagnostic Pitfalls in Guillain-Barré Syndrome: Case Report and Literature Review.Children (Basel). 2022 Dec 15;9(12):1969. doi: 10.3390/children9121969. Children (Basel). 2022. PMID: 36553412 Free PMC article. Review.
-
[COVID-19 vaccine safety: results of active surveillance at a sentinel site in ArgentinaSegurança das vacinas contra COVID-19: resultados da vigilância ativa em uma unidade sentinela da Argentina].Rev Panam Salud Publica. 2024 Dec 16;48:e94. doi: 10.26633/RPSP.2024.94. eCollection 2024. Rev Panam Salud Publica. 2024. PMID: 39687241 Free PMC article. Spanish.
-
Neurolymphomatosis mimicking a Guillain-Barré syndrome triggered by COVID-19 vaccination.Neuropathology. 2025 Feb;45(1):76-82. doi: 10.1111/neup.13003. Epub 2024 Sep 23. Neuropathology. 2025. PMID: 39311044 Free PMC article.
-
COVID-19 as a trigger of Guillain-Barré syndrome: A review of the molecular mechanism.Immun Inflamm Dis. 2023 May;11(5):e875. doi: 10.1002/iid3.875. Immun Inflamm Dis. 2023. PMID: 37249286 Free PMC article. Review.
-
Guillain-Barré syndrome associated with SARS-CoV-2 vaccination: how is it different? a systematic review and individual participant data meta-analysis.Clin Exp Vaccine Res. 2023 Apr;12(2):143-155. doi: 10.7774/cevr.2023.12.2.143. Epub 2023 Apr 30. Clin Exp Vaccine Res. 2023. PMID: 37214140 Free PMC article.
References
-
- Rosenblum H.G., et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the Advisory Committee on Immunization Practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1094–1099. - PMC - PubMed
-
- Fokke C., van den Berg B., Drenthen J., Walgaard C., van Doorn P.A., Jacobs B.C. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. 2014;137(1):33–43. - PubMed
-
- Greene S.K., Rett M., Weintraub E.S., Li L., Yin R., Amato A.A., et al. Risk of confirmed Guillain-Barre syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009–2010. Am J Epidemiol. 2012;175(11):1100–1109. - PMC - PubMed
-
- Stratton K., et al. Institute of Medicine (US) Immunization Safety Review Committee; 2004. Immunization safety review: influenza vaccines and neurological complications. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

