Transplantation of PSC-derived myogenic progenitors counteracts disease phenotypes in FSHD mice
- PMID: 36056021
- PMCID: PMC9440030
- DOI: 10.1038/s41536-022-00249-0
Transplantation of PSC-derived myogenic progenitors counteracts disease phenotypes in FSHD mice
Abstract
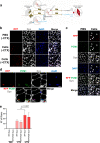
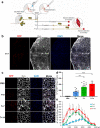
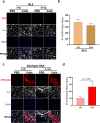
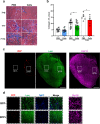
Facioscapulohumeral muscular dystrophy (FSHD) is a genetically dominant progressive myopathy caused by improper silencing of the DUX4 gene, leading to fibrosis, muscle atrophy, and fatty replacement. Approaches focused on muscle regeneration through the delivery of stem cells represent an attractive therapeutic option for muscular dystrophies. To investigate the potential for cell transplantation in FSHD, we have used the doxycycline-regulated iDUX4pA-HSA mouse model in which low-level DUX4 can be induced in skeletal muscle. We find that mouse pluripotent stem cell (PSC)-derived myogenic progenitors engraft in muscle actively undergoing DUX4-mediated degeneration. Donor-derived muscle tissue displayed reduced fibrosis and importantly, engrafted muscles showed improved contractile specific force compared to non-transplanted controls. These data demonstrate the feasibility of replacement of diseased muscle with PSC-derived myogenic progenitors in a mouse model for FSHD, and highlight the potential for the clinical benefit of such a cell therapy approach.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

