Low quality antibody responses in critically ill patients hospitalized with pandemic influenza A(H1N1)pdm09 virus infection
- PMID: 36056075
- PMCID: PMC9440095
- DOI: 10.1038/s41598-022-18977-0
Low quality antibody responses in critically ill patients hospitalized with pandemic influenza A(H1N1)pdm09 virus infection
Abstract
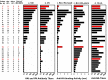
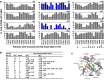
Although some adults infected with influenza 2009 A(H1N1)pdm09 viruses mounted high hemagglutination inhibition (HAI) antibody response, they still suffered from severe disease, or even death. Here, we analyzed antibody profiles in patients (n = 31, 17-65 years) admitted to intensive care units (ICUs) with lung failure and invasive mechanical ventilation use due to infection with A(H1N1)pdm09 viruses during 2009-2011. We performed a comprehensive analysis of the quality and quantity of antibody responses using HAI, virus neutralization, biolayer interferometry, enzyme-linked-lectin and enzyme-linked immunosorbent assays. At time of the ICU admission, 45% (14/31) of the patients had HAI antibody titers ≥ 80 in the first serum (S1), most (13/14) exhibited narrowly-focused HAI and/or anti-HA-head binding antibodies targeting single epitopes in or around the receptor binding site. In contrast, 42% (13/31) of the patients with HAI titers ≤ 10 in S1 had non-neutralizing anti-HA-stem antibodies against A(H1N1)pdm09 viruses. Only 19% (6/31) of the patients showed HA-specific IgG1-dominant antibody responses. Three of 5 fatal patients possessed highly focused cross-type HAI antibodies targeting the (K130 + Q223)-epitopes with extremely low avidity. Our findings suggest that narrowly-focused low-quality antibody responses targeting specific HA-epitopes may have contributed to severe infection of the lower respiratory tract.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

