Redox regulation of the immune response
- PMID: 36056148
- PMCID: PMC9508259
- DOI: 10.1038/s41423-022-00902-0
Redox regulation of the immune response
Abstract
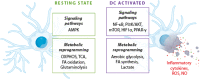
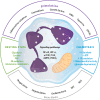
The immune-inflammatory response is associated with increased nitro-oxidative stress. The aim of this mechanistic review is to examine: (a) the role of redox-sensitive transcription factors and enzymes, ROS/RNS production, and the activity of cellular antioxidants in the activation and performance of macrophages, dendritic cells, neutrophils, T-cells, B-cells, and natural killer cells; (b) the involvement of high-density lipoprotein (HDL), apolipoprotein A1 (ApoA1), paraoxonase-1 (PON1), and oxidized phospholipids in regulating the immune response; and (c) the detrimental effects of hypernitrosylation and chronic nitro-oxidative stress on the immune response. The redox changes during immune-inflammatory responses are orchestrated by the actions of nuclear factor-κB, HIF1α, the mechanistic target of rapamycin, the phosphatidylinositol 3-kinase/protein kinase B signaling pathway, mitogen-activated protein kinases, 5' AMP-activated protein kinase, and peroxisome proliferator-activated receptor. The performance and survival of individual immune cells is under redox control and depends on intracellular and extracellular levels of ROS/RNS. They are heavily influenced by cellular antioxidants including the glutathione and thioredoxin systems, nuclear factor erythroid 2-related factor 2, and the HDL/ApoA1/PON1 complex. Chronic nitro-oxidative stress and hypernitrosylation inhibit the activity of those antioxidant systems, the tricarboxylic acid cycle, mitochondrial functions, and the metabolism of immune cells. In conclusion, redox-associated mechanisms modulate metabolic reprogramming of immune cells, macrophage and T helper cell polarization, phagocytosis, production of pro- versus anti-inflammatory cytokines, immune training and tolerance, chemotaxis, pathogen sensing, antiviral and antibacterial effects, Toll-like receptor activity, and endotoxin tolerance.
Keywords: Antioxidants; Immune response; Inflammation; Oxidative and nitrosative stress; Physiological stress.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

