LRP1 is a neuronal receptor for α-synuclein uptake and spread
- PMID: 36056345
- PMCID: PMC9438229
- DOI: 10.1186/s13024-022-00560-w
LRP1 is a neuronal receptor for α-synuclein uptake and spread
Abstract
Background: The aggregation and spread of α-synuclein (α-Syn) protein and related neuronal toxicity are the key pathological features of Parkinson's disease (PD) and Lewy body dementia (LBD). Studies have shown that pathological species of α-Syn and tau can spread in a prion-like manner between neurons, although these two proteins have distinct pathological roles and contribute to different neurodegenerative diseases. It is reported that the low-density lipoprotein receptor-related protein 1 (LRP1) regulates the spread of tau proteins; however, the molecular regulatory mechanisms of α-Syn uptake and spread, and whether it is also regulated by LRP1, remain poorly understood.
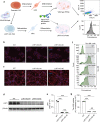
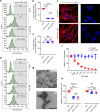
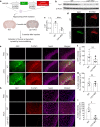
Methods: We established LRP1 knockout (LRP1-KO) human induced pluripotent stem cells (iPSCs) isogenic lines using a CRISPR/Cas9 strategy and generated iPSC-derived neurons (iPSNs) to test the role of LRP1 in α-Syn uptake. We treated the iPSNs with fluorescently labeled α-Syn protein and measured the internalization of α-Syn using flow cytometry. Three forms of α-Syn species were tested: monomers, oligomers, and pre-formed fibrils (PFFs). To examine whether the lysine residues of α-Syn are involved in LRP1-mediated uptake, we capped the amines of lysines on α-Syn with sulfo-NHS acetate and then measured the internalization. We also tested whether the N-terminus of α-Syn is critical for LRP1-mediated internalization. Lastly, we investigated the role of Lrp1 in regulating α-Syn spread with a neuronal Lrp1 conditional knockout (Lrp1-nKO) mouse model. We generated adeno-associated viruses (AAVs) that allowed for distinguishing the α-Syn expression versus spread and injected them into the hippocampus of six-month-old Lrp1-nKO mice and the littermate wild type (WT) controls. The spread of α-Syn was evaluated three months after the injection.
Results: We found that the uptake of both monomeric and oligomeric α-Syn was significantly reduced in iPSNs with LRP1-KO compared with the WT controls. The uptake of α-Syn PFFs was also inhibited in LRP1-KO iPSNs, albeit to a much lesser extent compared to α-Syn monomers and oligomers. The blocking of lysine residues on α-Syn effectively decreased the uptake of α-Syn in iPSNs and the N-terminus of α-Syn was critical for LRP1-mediated α-Syn uptake. Finally, in the Lrp1-nKO mice, the spread of α-Syn was significantly reduced compared with the WT littermates.
Conclusions: We identified LRP1 as a key regulator of α-Syn neuronal uptake, as well as an important mediator of α-Syn spread in the brain. This study provides new knowledge on the physiological and pathological role of LRP1 in α-Syn trafficking and pathology, offering insight for the treatment of synucleinopathies.
Keywords: Human induced pluripotent stem cells; Lewy body dementia; Low-density lipoprotein receptor-related protein 1; Parkinson’s disease; α-Synuclein.
© 2022. The Author(s).
Conflict of interest statement
G.B. consults for SciNeuro, has consulted for AbbVie, E-Scape, Eisai, and Vida Ventures and is on the scientific advisory board for Kisbee Therapeutics. D.M.H. is as an inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies. D.M.H. co-founded and is on the scientific advisory board of C2N Diagnostics. D.M.H. is on the scientific advisory board of Genentech, Denali and Cajal Neuroscience and consults for Alector. All other authors declare no competing interests.
Figures

References
-
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of α-Synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

