Enhancing COVID Rehabilitation with Technology (ECORT): protocol for an open-label, single-site randomized controlled trial evaluating the effectiveness of electronic case management for individuals with persistent COVID-19 symptoms
- PMID: 36056372
- PMCID: PMC9437413
- DOI: 10.1186/s13063-022-06578-1
Enhancing COVID Rehabilitation with Technology (ECORT): protocol for an open-label, single-site randomized controlled trial evaluating the effectiveness of electronic case management for individuals with persistent COVID-19 symptoms
Abstract
Background: As of May 2022, Ontario has seen more than 1.3 million cases of COVID-19. While the majority of individuals will recover from infection within 4 weeks, a significant subset experience persistent and often debilitating symptoms, known as "post-COVID syndrome" or "Long COVID." Those with Long COVID experience a wide array of symptoms, with variable severity, including fatigue, cognitive impairment, and shortness of breath. Further, the prevalence and duration of Long COVID is not clear, nor is there evidence on the best course of rehabilitation for individuals to return to their desired level of function. Previous work with chronic conditions has suggested that the addition of electronic case management (ECM) may help to improve outcomes. These platforms provide enhanced connection with care providers, detailed symptom tracking and goal setting, and access to relevant resources. In this study, our primary aim is to determine if the addition of ECM with health coaching improves Long COVID outcomes at 3 months compared to health coaching alone.
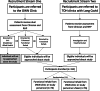
Methods: The trial is an open-label, single-site, randomized controlled trial of ECM with health coaching (ECM+) compared to health coaching alone (HC). Both groups will continue to receive usual care. Participants will be randomized equally to receive health coaching (± ECM) for a period of 8 weeks and a 12-week follow-up. Our primary outcome is the WHO Disability Assessment Scale (WHODAS), 36-item self-report total score. Participants will also complete measures of cognition, fatigue, breathlessness, and mental health. Participants and care providers will be asked to complete a brief qualitative interview at the end of the study to evaluate acceptability and implementation of the intervention.
Discussion: There is currently little evidence about the optimal treatment of Long COVID patients or the use of digital health platforms in this population. The results of this trial could result in rapid, scalable, and personalized care for people with Long COVID which will decrease morbidity after an acute infection. Results from this study will also inform decision making in Long COVID and treatment guidelines at provincial and national levels.
Trial registration: ClinicalTrials.gov NCT05019963. Registered on 25 August 2021.
Keywords: Blended care; COVID-19; SARS-CoV-2; Long COVID; Electronic case management; Post-COVID rehabilitation.
© 2022. The Author(s).
Conflict of interest statement
MK has consulting or speaking fees, unrelated to this trial or intervention, from HLS, Janssen, Otsuka, and Lundbeck in the last 2 years. TM, unrelated to this trial or intervention, has been a consultant for CHDI, Sunovion, Valeo Pharma, Roche, Biogen, and nQ Medical and has been involved in Speakers Bureaus for Abbvie and Valeo Pharma.
The other authors declare that they have no competing interests.
References
-
- Government of Ontario . All Ontario: case numbers and spread. 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous