Natural history comparison study to assess the efficacy of elamipretide in patients with Barth syndrome
- PMID: 36056411
- PMCID: PMC9438322
- DOI: 10.1186/s13023-022-02469-5
Natural history comparison study to assess the efficacy of elamipretide in patients with Barth syndrome
Abstract
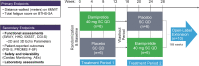
Background: Natural history studies are increasingly recognized as having an important role in drug development for rare diseases. A phase 3, observational, retrospective, and non-interventional study was designed to establish a natural history control (NHC) cohort of patients with Barth syndrome (BTHS) to provide further analysis of the efficacy of elamipretide observed in an open-label extension (OLE) phase of the TAZPOWER trial, a clinical trial that tested the efficacy of 40 mg daily of elamipretide in patients with BTHS.
Methods: This was a retrospective, non-interventional study. A propensity score model was used to compare elamipretide-treated patients and NHCs. The analysis included 8 patients from the TAZPOWER OLE and 19 untreated NHCs (including 12 with serial echocardiographic assessments).
Results: For the 6-min walk test (6MWT, primary endpoint), the least squares (LS) mean difference between groups was 79.7 m (P = 0.0004) at week 64 and 91.0 m (P = 0.0005) at week 76 in favor of elamipretide. Significant improvements in muscle strength (secondary endpoint), as assessed by handheld dynamometry (HHD) were also observed with elamipretide, with LS mean differences of 40.8 Newtons at 64 weeks (P = 0.0002) and 56.7 Newtons at 76 weeks (P = 0.0005). Patients continuously treated with elamipretide also experienced statistically significant improvements in other secondary endpoints (i.e., 5 times sit-to-stand [5XSST], multi-domain responder index [MDRI]). The functional improvements were robust to sensitivity analyses. Left ventricular stroke volume increased from baseline in patients with elamipretide but decreased in NHCs.
Conclusions: Overall, the study established a NHC for use in assessing the efficacy of therapeutic interventions in patients with BTHS and the results suggest that elamipretide may improve natural history of BTHS at least in part by attenuating the natural decline in heart function and provide meaningful improvements in heart function and functional capacity in patients with BTHS compared to NHCs.
Highlights: A matched Natural History Control (NHC) was used to evaluate elamipretide in BTHS Elamipretide may improve natural history of BTHS by attenuating natural decline in heart function Elamipretide was associated with meaningful clinical improvements in skeletal muscle and cardiovascular parameters that were not observed in NHCs The study established a NHC for use in assessing the efficacy of therapeutic interventions in BTHS.
Keywords: Barth syndrome; Elamipretide; Natural history control; TAZPOWER.
© 2022. The Author(s).
Conflict of interest statement
AA and JC are employees of Stealth BioTherapeutics, Needham, MA. HJV received clinical trial and research funding from Stealth BioTherapeutics, Needham, MA.
Figures
References
-
- Ferreira C, Thompson R, and Vernon H (1993) Barth syndrome. In GeneReviews((R)) (Adam M P, Ardinger H H, Pagon R A, Wallace S E, Bean L J H, Stephens K, and Amemiya A, eds), Seattle (WA)
-
- Rigaud C, Lebre AS, Touraine R, Beaupain B, Ottolenghi C, Chabli A, Ansquer H, Ozsahin H, Di Filippo S, De Lonlay P, Borm B, Rivier F, Vaillant MC, Mathieu-Dramard M, Goldenberg A, Viot G, Charron P, Rio M, Bonnet D, Donadieu J. Natural history of barth syndrome: a national cohort study of 22 patients. Orphanet J Rare Dis. 2013;8:70. doi: 10.1186/1750-1172-8-70. - DOI - PMC - PubMed
-
- D'Adamo P, Fassone L, Gedeon A, Janssen EA, Bione S, Bolhuis PA, Barth PG, Wilson M, Haan E, Orstavik KH, Patton MA, Green AJ, Zammarchi E, Donati MA, Toniolo D. The X-linked gene G4.5 is responsible for different infantile dilated cardiomyopathies. Am J Hum Genet. 1997;61:862–867. doi: 10.1086/514886. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

