CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma
- PMID: 36056553
- PMCID: PMC9840126
- DOI: 10.1016/j.ymthe.2022.08.021
CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma
Abstract
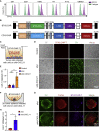
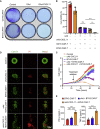
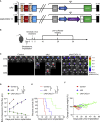
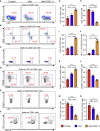
Glioblastoma (GBM) is the most aggressive primary malignant brain cancer and urgently requires effective treatments. Chimeric antigen receptor T (CAR-T) cell therapy offers a potential treatment method, but it is often hindered by poor infiltration of CAR-T cells in tumors and highly immunosuppressive tumor microenvironment (TME). Here, we armed an oncolytic adenovirus (oAds) with a chemokine CXCL11 to increase the infiltration of CAR-T cells and reprogram the immunosuppressive TME, thus improving its therapeutic efficacy. In both immunodeficient and immunocompetent orthotopic GBM mice models, we showed that B7H3-targeted CAR-T cells alone failed to inhibit GBM growth but, when combined with the intratumoral administration of CXCL11-armed oAd, it achieved a durable antitumor response. Besides, oAd-CXCL11 had a potent antitumor effect and reprogramed the immunosuppressive TME in GL261 GBM models, in which increased infiltration of CD8+ T lymphocytes, natural killer (NK) cells, and M1-polarized macrophages, while decreased proportions of myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and M2-polarized macrophages were observed. Furthermore, the antitumor effect of the oAd-CXCL11 was CD8+ T cell dependent. Our findings thus revealed that CXCL11-armed oAd can improve immune-virotherapy and can be a promising adjuvant of CAR-T therapy for GBM.
Keywords: CAR-T; GBM; chemokine; combination therapy; oncolytic virus; tumor microenvironment.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.W., A.T., and L.Z. have filed patents related to this work. The other authors declare no competing interests.
Figures




References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. - PubMed
-
- Tan A.C., Ashley D.M., López G.Y., Malinzak M., Friedman H.S., Khasraw M. Management of glioblastoma: state of the art and future directions. CA. Cancer J. Clin. 2020;70:299–312. - PubMed
-
- Medikonda R., Dunn G., Rahman M., Fecci P., Lim M. A review of glioblastoma immunotherapy. J. Neurooncol. 2021;151:41–53. - PubMed
-
- Johnson P.C., Abramson J.S. Engineered T cells: CAR T cell therapy and beyond. Curr. Oncol. Rep. 2022;24:23–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

