Immunogenicity of two doses of BNT162b2 and mRNA-1273 vaccines for solid cancer patients on treatment with or without a previous SARS-CoV-2 infection
- PMID: 36056571
- PMCID: PMC9538813
- DOI: 10.1002/ijc.34273
Immunogenicity of two doses of BNT162b2 and mRNA-1273 vaccines for solid cancer patients on treatment with or without a previous SARS-CoV-2 infection
Abstract
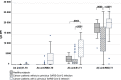
Previous studies on the immunogenicity of SARS-CoV-2 mRNA vaccines showed a reduced seroconversion in cancer patients. The aim of our study is to evaluate the immunogenicity of two doses of mRNA vaccines in solid cancer patients with or without a previous exposure to the virus. This is a single-institution, prospective, nonrandomized study. Patients in active treatment and a control cohort of healthy people received two doses of BNT162b2 (Comirnaty, BioNTech/Pfizer, The United States) or mRNA-1273 (Spikevax, Moderna). Vaccine was administered before starting anticancer therapy or on the first day of the treatment cycle. SARS-CoV-2 antibody levels against S1, RBD (to evaluate vaccine response) and N proteins (to evaluate previous infection) were measured in plasma before the first dose and 30 days after the second one. From January to June 2021, 195 consecutive cancer patients and 20 healthy controls were enrolled. Thirty-one cancer patients had a previous exposure to SARS-CoV-2. Cancer patients previously exposed to the virus had significantly higher median levels of anti-S1 and anti-RBD IgG, compared to healthy controls (P = .0349) and to cancer patients without a previous infection (P < .001). Vaccine type (anti-S1: P < .0001; anti-RBD: P = .0045), comorbidities (anti-S1: P = .0274; anti-RBD: P = .0048) and the use of G-CSF (anti-S1: P = .0151) negatively affected the antibody response. Conversely, previous exposure to SARS-CoV-2 significantly enhanced the response to vaccination (anti-S1: P < .0001; anti-RBD: P = .0026). Vaccine immunogenicity in cancer patients with a previous exposure to SARS-CoV-2 seems comparable to that of healthy subjects. On the other hand, clinical variables of immune frailty negatively affect humoral immune response to vaccination.
Keywords: COVID-19; cancer patients; immunogenicity; mRNA; vaccines.
© 2022 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Nicla La Verde reports grant from Eisai; speaker bureau from GSK, travel expenses for conference from Gentili, Celgene, Pfizer; advisory role from Novartis and Celgene; advisor role, travel expenses for conference from Pfizer; advisory board from MSD, Roche, Novartis, Astrazeneca. Davide Dalu reports receiving grants from Gentili, travel expenses from Roche, Gentili, Eisai. There are no other personal or financial conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

