Clinical autonomic nervous system laboratories in Europe: A joint survey of the European Academy of Neurology and the European Federation of Autonomic Societies: A joint survey of the European Academy of Neurology and the European Federation of Autonomic Societies
- PMID: 36056590
- PMCID: PMC9826284
- DOI: 10.1111/ene.15538
Clinical autonomic nervous system laboratories in Europe: A joint survey of the European Academy of Neurology and the European Federation of Autonomic Societies: A joint survey of the European Academy of Neurology and the European Federation of Autonomic Societies
Abstract
Background and purpose: Disorders of the autonomic nervous system (ANS) are common conditions, but it is unclear whether access to ANS healthcare provision is homogeneous across European countries. The aim of this study was to identify neurology-driven or interdisciplinary clinical ANS laboratories in Europe, describe their characteristics and explore regional differences.
Methods: We contacted the European national ANS and neurological societies, as well as members of our professional network, to identify clinical ANS laboratories in each country and invite them to answer a web-based survey.
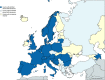
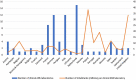
Results: We identified 84 laboratories in 22 countries and 46 (55%) answered the survey. All laboratories perform cardiovascular autonomic function tests, and 83% also perform sweat tests. Testing for catecholamines and autoantibodies are performed in 63% and 56% of laboratories, and epidermal nerve fiber density analysis in 63%. Each laboratory is staffed by a median of two consultants, one resident, one technician and one nurse. The median (interquartile range [IQR]) number of head-up tilt tests/laboratory/year is 105 (49-251). Reflex syncope and neurogenic orthostatic hypotension are the most frequently diagnosed cardiovascular ANS disorders. Thirty-five centers (76%) have an ANS outpatient clinic, with a median (IQR) of 200 (100-360) outpatient visits/year; 42 centers (91%) also offer inpatient care (median 20 [IQR 4-110] inpatient stays/year). Forty-one laboratories (89%) are involved in research activities. We observed a significant difference in the geographical distribution of ANS services among European regions: 11 out of 12 countries from North/West Europe have at least one ANS laboratory versus 11 out of 21 from South/East/Greater Europe (p = 0.021).
Conclusions: This survey highlights disparities in the availability of healthcare services for people with ANS disorders across European countries, stressing the need for improved access to specialized care in South, East and Greater Europe.
Keywords: (disorders of) autonomic nervous system; Composite Autonomic Severity Score; Survey; cardiovascular autonomic function tests; disorders of consciousness (other than epilepsy); neurodisparity; neurological disorders; orthostatic hypotension; sweat tests; syncope.
© 2022 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Mario Habek: participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals and TG Pharmaceuticals, outside of the present work. Fabian Leys: none. Dr Leys is supported by grants from the US MSA Coalition and Dr Johannes and Herta Tuba Foundation. Magdalena Krbot Skorić: received consultation and/or speaker fees from Sanofi Genzyme and Roche, outside of the present work. Diogo Reis Carneiro: received speaker fees from Almirall, outside of the present work. Giovanna Calandra‐Buonaura: received honoraria for speaking engagements or consulting activities from Abbvie, Bial and Zambon, outside the present work. Jennifer Camaradou: participated as a costed PPI Patient Public Involvement lead in a study at University College London funded by the UK National Institute for Health Research (NIHR)132914 and UK NIHR20334 at Bristol UWE, received consultation fees from Roche Canada, Roche Global, Medable Inc, GSK Glaxco Smith Klein, outside of the present work, and received small honoraria as a lay member of the UK NICE Covid expert panel, as a Citizen Partner COVID END, Evidence Synthesis network (WHO COVID‐19 evidence collaborative partner) and as a lay member on the NIHR AI AWARD panel, and is a Cochrane Convenes citizen partner, Patient Editor Advances in Therapy Journal Springer Health, a patient representative on UK MRC ADPD Advanced Pain Discovery Platform and an ambassador for the OneNeurology Global Partnership for EMEA. Giacomo Chiaro: none. Pietro Cortelli: reports no financial disclosure. Cristian Falup‐Pecurariu: received royalties from Elsevier, Springer Verlag, honoraria from Abbvie, International Parkinson Disease and Movement Disorders Society, outside of the present work. Roberta Granata: none. Pietro Guaraldi: has been an advisory board member of Alnylam and Sobi; received speaker fees and honoraria from Theravance Biopharma, Akcea Therapeutics; Alnylam and Chiesi, received congress and travel accommodation expense compensations from Alnylam, Bial, Zambon, Sobi and Abbvie, all outside of the present work. Raimund Helbok: none. Max J. Hilz: received consultancy and speaker fees from Sanofi Genzyme, consultancy fees from Pfizer, and editor honoraria from Elsevier BV, outside of the present work. Valeria Iodice: reports speaker fees and honoraria from Theravance Biopharma, Janssen, outside of the present work. Jens Jordan: served as advisor for Novo‐Nordisk, received research support from Boehringer‐Ingelheim and Novo‐Nordisk, and is co‐founder of Eternygen GmbH. Evert C. A. Kaal: none. Anita Kamondi: none. Anne Pavy Le Traon: reports honoraria from Biohaven outside of the present work. Isabel Rocha: nothing to disclose. Johann Sellner: none related to this work. Jean Michel Senard: none. Astrid Terkelsen: received consultation and/or speaker fees from Alnylam Pharmaceuticals, Akcea Therapeutics, Pfizer and Allergan, however, without relation to the present work. Gregor K. Wenning: reports consultancy and lecture fees from AbbVie, AFFiRiS AG, AstraZeneca, Biogen, Biohaven, Inhibicase, Lundbeck, Merz, Ono, Teva and Theravance, and research grants from the Austrian Science Fund (FWF), the Austrian National Bank, the US MSA Coalition, Parkinson Fonds Austria, the Dr Johannes und Hertha Tuba Foundation, and the International Parkinson and Movement Disorder Society, outside of the submitted work. Thomas Berger: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, GSK, GWD/Jazz Pharma, Horizon, Janssen‐Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi‐Genzyme, Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Sanofi Aventis, Teva) and for participation in clinical trials in multiple sclerosis and related disorders sponsored by Alexion, Bayer, Biogen, BMS/Celgene, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, Teva. Roland Thijs: reports research support from the ‘Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie’, Michael J Fox Foundation, Human Measurement Models Programme co‐funded by Health~Holland, Top Sector Life Sciences & Health and ZonMw under grant agreement 114,025,101 (Brain@Home) and speaker or consultant fees from Theravance Biopharma, Arvelle, Medtronic, Zogenix, UCB and NewLife Wearables. Walter Struhal: none. Alessandra Fanciulli: reports royalties from Springer Verlag, speaker fees and honoraria from Theravance Biopharma, Abbvie, Healthware, the International Parkinson Disease and Movement Disorders Society, the Austrian Neurology Society, the Austrian Autonomic Society and research grants from the Parkinson Fond, US MSA Coalition, Dr Johannes and Hertha Tuba Foundation and Austrian Exchange Program, outside of the present work.
Figures
References
-
- Kaufmann H, Norcliffe‐Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med. 2020;382(2):163‐178. - PubMed
-
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878‐885. - PubMed
-
- Cheshire WP, Freeman R, Gibbons CH, et al. Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American autonomic society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132(2):666‐682. - PubMed
-
- Lahrmann H, Magnifico F, Haensch CA, Cortelli P. Autonomic nervous system laboratories: a European survey. Eur J Neurol. 2005;12(5):375‐379. - PubMed
-
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69‐72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

