COVID-19 immunopathology: From acute diseases to chronic sequelae
- PMID: 36056655
- PMCID: PMC9537925
- DOI: 10.1002/jmv.28122
COVID-19 immunopathology: From acute diseases to chronic sequelae
Abstract
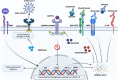
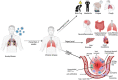
The clinical manifestation of coronavirus disease 2019 (COVID-19) mainly targets the lung as a primary affected organ, which is also a critical site of immune cell activation by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, recent reports also suggest the involvement of extrapulmonary tissues in COVID-19 pathology. The interplay of both innate and adaptive immune responses is key to COVID-19 management. As a result, a robust innate immune response provides the first line of defense, concomitantly, adaptive immunity neutralizes the infection and builds memory for long-term protection. However, dysregulated immunity, both innate and adaptive, can skew towards immunopathology both in acute and chronic cases. Here we have summarized some of the recent findings that provide critical insight into the immunopathology caused by SARS-CoV-2, in acute and post-acute cases. Finally, we further discuss some of the immunomodulatory drugs in preclinical and clinical trials for dampening the immunopathology caused by COVID-19.
Keywords: SARS coronavirus; immnopathology; immune responses; respiratory tract.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
Jie Sun is a consultant for TeneoFour company.
Figures


References
-
- COVID‐19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed September 7, 2022. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

