Food and Drug Administration Approval of Artesunate for Severe Malaria: Enough to Achieve Best Practice?
- PMID: 36056897
- PMCID: PMC10169404
- DOI: 10.1093/cid/ciac728
Food and Drug Administration Approval of Artesunate for Severe Malaria: Enough to Achieve Best Practice?
Abstract
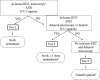
Intravenous artesunate has been the global standard of care for severe malaria for over 2 decades. Yet, until recently, artesunate has only been available to patients through an expanded-access protocol from the Centers for Disease Control and Prevention. In May 2020, the Food and Drug Administration approved artesunate, allowing US hospitals to stock the drug and ensuring prompt treatment for this life-threatening infection. However, because of artesunate's high cost and the infrequency of severe malaria in the United States, hospitals may be reluctant to stock the drug. As US health systems weigh the decision to stock artesunate, we propose a hospital tier framework to inform this decision and support clinicians caring for patients who present with severe malaria.
Keywords: artesunate; artesunate access; severe malaria.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. W. M. S. receives partial chapter royalties from UpToDate; acts as a consultant for BCTPartners (minority-owned business aimed at increasing business/healthcare diversity and equity); and received a one-time honorarium from Haymarket Medical Education (development of an open-access, noncommercial CME module). J. D. A. received funding from Arnold Ventures for research on off-patent drug pricing through the end of 2020 and from the Centers for Disease Control and Prevention as co-investigator on the Vaccine Safety Datalink (VSD; ended June 2022). C. M. T. reports no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Centers for Disease Control and Prevention. FDA approval of artesunate for infection for treatment of severe malaria . 2020. Available at: https://www.cdc.gov/malaria/new_info/2020/artesunate_approval.html#:∼:te.... Accessed 6 July 2022.
-
- Centers for Disease Control and Prevention. Intravenous artesunate for treatment of severe malaria in the United States . Available at: https://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html#:∼:text=.... Accessed 6 July 2022.
-
- Food and Drug Administration. Treatment of uncomplicated Plasmodium falciparum malaria. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021799s000_MedR_.... Accessed 6 July 2022.
-
- Centers for Disease Control and Prevention. Expanded access IND protocol: use of intravenous artesunate for treatment of severe malaria in the United States . 2019. Available at: https://www.cdc.gov/malaria/resources/pdf/guilin_protocol_7171_guilin_iv.... Accessed 10 April 2022.
-
- Amivas LLC. Reporting required by manufacturers of new high-cost prescription drugs . 2021. Available at: https://ago.vermont.gov/wp-content/uploads/2021/10/Vermont-Notification_.... Accessed 25 March 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

