Clinical effect of early administration of tocilizumab following the initiation of corticosteroid therapy for patients with COVID-19
- PMID: 36057415
- PMCID: PMC9428329
- DOI: 10.1016/j.jiac.2022.08.021
Clinical effect of early administration of tocilizumab following the initiation of corticosteroid therapy for patients with COVID-19
Abstract
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first broke out in Wuhan in December 2019, and has since caused a global pandemic. The efficacy of several drugs has been evaluated, and it is now evident that tocilizumab has a beneficial effect, especially combined with corticosteroids, in patients with Coronavirus Disease 2019 (COVID-19). However, the optimal timing of tocilizumab administration has not yet been established. The goal of the present study was to determine the optimal timing of tocilizumab administration after starting corticosteroid therapy in patients with COVID-19.
Methods: We retrospectively analyzed the clinical characteristics of patients who were hospitalized for COVID-19 and treated with tocilizumab and corticosteroids in our hospital. The patients were divided into concurrent and sequential groups. The concurrent group received tocilizumab ≤24 h after corticosteroids, and the sequential group received tocilizumab >24 h after corticosteroid administration.
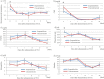
Results: The baseline clinical characteristics of tocilizumab administration were similar between the two groups. White blood cell counts were significantly lower and C-reactive protein levels were significantly higher in the concurrent group than the sequential group. In the concurrent group, tocilizumab administration led to a significant decrease in maximum body temperature. In addition, there were significantly more oxygen-free days in the concurrent group than in the sequential group. However, survival rate was not significantly different between the concurrent and the sequential groups.
Conclusions: In the combination therapy with tocilizumab and corticosteroids, early administration of tocilizumab after starting corticosteroid treatment is effective when treating COVID-19.
Keywords: COVID-19; Corticosteroids; SARS-CoV-2; Tocilizumab.
Copyright © 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

