Predictive performance and clinical application of COV50, a urinary proteomic biomarker in early COVID-19 infection: a prospective multicentre cohort study
- PMID: 36057526
- PMCID: PMC9432869
- DOI: 10.1016/S2589-7500(22)00150-9
Predictive performance and clinical application of COV50, a urinary proteomic biomarker in early COVID-19 infection: a prospective multicentre cohort study
Erratum in
-
Correction to Lancet Digit Health 2022; 4: e727-37.Lancet Digit Health. 2023 Feb;5(2):e58. doi: 10.1016/S2589-7500(22)00248-5. Epub 2022 Dec 15. Lancet Digit Health. 2023. PMID: 36528543 Free PMC article. No abstract available.
Abstract
Background: The SARS-CoV-2 pandemic is a worldwide challenge. The CRIT-CoV-U pilot study generated a urinary proteomic biomarker consisting of 50 peptides (COV50), which predicted death and disease progression from SARS-CoV-2. After the interim analysis presented for the German Government, here, we aimed to analyse the full dataset to consolidate the findings and propose potential clinical applications of this biomarker.
Methods: CRIT-CoV-U was a prospective multicentre cohort study. In eight European countries (Austria, France, Germany, Greece, North Macedonia, Poland, Spain, and Sweden), 1012 adults with PCR-confirmed COVID-19 were followed up for death and progression along the 8-point WHO scale. Capillary electrophoresis coupled with mass spectrometry was used for urinary proteomic profiling. Statistical methods included logistic regression and receiver operating characteristic curve analysis with a comparison of the area under curve (AUC) between nested models. Hospitalisation costs were derived from the care facility corresponding with the Markov chain probability of reaching WHO scores ranging from 3 to 8 and flat-rate hospitalisation costs adjusted for the gross per capita domestic product of each country.
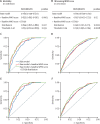
Findings: From June 30 to Nov 19, 2020, 228 participants were recruited, and from April 30, 2020, to April 14, 2021, 784 participants were recruited, resulting in a total of 1012 participants. The entry WHO scores were 1-3 in 445 (44%) participants, 4-5 in 529 (52%) participants, and 6 in 38 (4%) participants; and of all participants, 119 died and 271 had disease progression. The odds ratio (OR) associated with COV50 in all 1012 participants for death was 2·44 (95% CI 2·05-2·92) unadjusted and 1·67 (1·34-2·07) when adjusted for sex, age, BMI, comorbidities, and baseline WHO score; and for disease progression, the OR was 1·79 (1·60-2·01) when unadjusted and 1·63 (1·41-1·91) when adjusted (p<0·0001 for all). The predictive accuracy of the optimised COV50 thresholds was 74·4% (71·6-77·1%) for mortality (threshold 0·47) and 67·4% (64·4-70·3%) for disease progression (threshold 0·04). When adjusted for covariables and the baseline WHO score, these thresholds improved AUCs from 0·835 to 0·853 (p=0·033) for death and from 0·697 to 0·730 (p=0·0008) for progression. Of 196 participants who received ambulatory care, 194 (99%) did not reach the 0·04 threshold. The cost reductions associated with 1 day less hospitalisation per 1000 participants were million Euro (M€) 0·887 (5-95% percentile interval 0·730-1·039) in participants at a low risk (COV50 <0·04) and M€2·098 (1·839-2·365) in participants at a high risk (COV50 ≥0·04).
Interpretation: The urinary proteomic COV50 marker might be predictive of adverse COVID-19 outcomes. Even in people with mild-to-moderate PCR-confirmed infections (WHO scores 1-4), the 0·04 COV50 threshold justifies earlier drug treatment, thereby potentially reducing the number of days in hospital and associated costs.
Funding: German Federal Ministry of Health.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests HM is the co-founder and co-owner of Mosaiques-Diagnostiques (Hannover, Germany). JS and JR are employees of Mosaiques-Diagnostics. HDR received consulting fees and honoraria for presentations from Alexion, AstraZeneca, and Bristol-Myers-Squibb. All other authors declare no competing interests.
Figures
References
-
- WHO Weekly epidemiological update on COVID-19—23 November 2021. Nov 23, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

