Preparation and DFT studies of chiral Cu (I)-complexes of biphenyl bisoxazolines and their application in enantioselective Kharasch-Sosnovsky reaction
- PMID: 36057728
- PMCID: PMC9440904
- DOI: 10.1038/s41598-022-18922-1
Preparation and DFT studies of chiral Cu (I)-complexes of biphenyl bisoxazolines and their application in enantioselective Kharasch-Sosnovsky reaction
Abstract
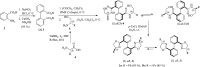
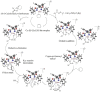
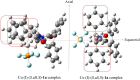
Effect of a range of t-butyl perbenzoates bearing electron-withdrawing and electron-donating substitutions on the phenyl ring and HZSM-5 as a porous additive at 0 °C in enantioselective allylic C-H bond oxidation of cyclic and acyclic olefins in the presence of Cu (I)-(S,aS,S) complexes of biphenyl bisoxazoline ligands, produced easily through the chelation-induced process, were investigated. The enantioenriched allylic esters were obtained in reasonable times with excellent enantioselectivities and yields using electron-withdrawing substituted peresters in the presence of Cu (I)-(S,aS,S)-1a complex, containing phenyl groups at the stereogenic centers of the oxazoline moieties. To reach a better insight on geometry, chemical activity, enantioselectivity, and thermodynamic stability of the Cu (I)-BOX complexes, DFT calculations with B3LYP-D3/6-31G (d, p) level of theory were applied to them. Moreover, NBO analysis was used to illustrate interactions between orbitals.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Chiral amido-oxazoline functionalized MCM-41: A sustainable heterogeneous catalyst for enantioselective Kharasch-Sosnovsky and Henry reactions.Heliyon. 2024 Oct 29;10(21):e39911. doi: 10.1016/j.heliyon.2024.e39911. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553607 Free PMC article.
-
Catalytic Allylic Oxidation of Alkenes Using an Asymmetric Kharasch-Sosnovsky Reaction.Angew Chem Int Ed Engl. 2001 Oct 1;40(19):3567-3571. doi: 10.1002/1521-3773(20011001)40:19<3567::AID-ANIE3567>3.0.CO;2-C. Angew Chem Int Ed Engl. 2001. PMID: 11592185
-
Studies on enantioselective allylic oxidation of olefins using peresters catalyzed by Cu(I)-complexes of chiral pybox ligands.Org Biomol Chem. 2006 Dec 7;4(23):4370-4. doi: 10.1039/b612423b. Epub 2006 Oct 24. Org Biomol Chem. 2006. PMID: 17102883
-
Theoretical insights into enantioselective catalysis: the mechanism of the Kharasch-Sosnovsky reaction.Chemistry. 2008;14(30):9274-85. doi: 10.1002/chem.200800638. Chemistry. 2008. PMID: 18756568
-
Enantioselective Allylic C-H Bond Oxidation of Olefins Using Copper Complexes of Chiral Oxazoline Based Ligands.Top Curr Chem (Cham). 2022 Mar 10;380(3):20. doi: 10.1007/s41061-022-00375-9. Top Curr Chem (Cham). 2022. PMID: 35274165 Review.
Cited by
-
Chiral amido-oxazoline functionalized MCM-41: A sustainable heterogeneous catalyst for enantioselective Kharasch-Sosnovsky and Henry reactions.Heliyon. 2024 Oct 29;10(21):e39911. doi: 10.1016/j.heliyon.2024.e39911. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553607 Free PMC article.
References
-
- Gómez M, Muller G, Rocamora M. Coordination chemistry of oxazoline ligands. Coord. Chem. Rev. 1999;193:769–835. doi: 10.1016/S0010-8545(99)00086-7. - DOI
-
- Fraile JM, García JI, Herrerías CI, Mayoral JA, Reiser O, Socuéllamos A, Werner H. The role of binding constants in the efficiency of chiral catalysts immobilized by electrostatic interactions: the case of azabis (oxazoline)–copper complexes. Chem. Eur. J. 2004;10:2997–3005. doi: 10.1002/chem.200305739. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

