Imaging of intestinal vasculitis focusing on MR and CT enterography: a two-way street between radiologic findings and clinical data
- PMID: 36057741
- PMCID: PMC9440973
- DOI: 10.1186/s13244-022-01284-7
Imaging of intestinal vasculitis focusing on MR and CT enterography: a two-way street between radiologic findings and clinical data
Abstract
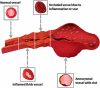
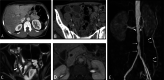
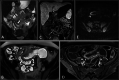
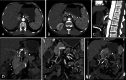
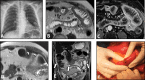
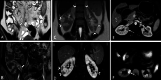
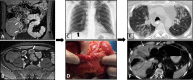
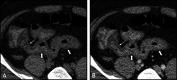
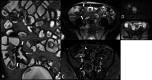
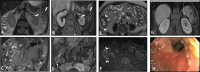
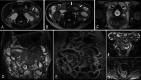
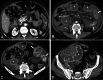
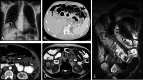
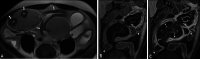
Diagnosis of intestinal vasculitis is often challenging due to the non-specific clinical and imaging findings. Vasculitides with gastrointestinal (GI) manifestations are rare, but their diagnosis holds immense significance as late or missed recognition can result in high mortality rates. Given the resemblance of radiologic findings with some other entities, GI vasculitis is often overlooked on small bowel studies done using computed tomography/magnetic resonance enterography (CTE/MRE). Hereon, we reviewed radiologic findings of vasculitis with gastrointestinal involvement on CTE and MRE. The variety of findings on MRE/CTE depend upon the size of the involved vessels. Signs of intestinal ischemia, e.g., mural thickening, submucosal edema, mural hyperenhancement, and restricted diffusion on diffusion-weighted imaging, are common in intestinal vasculitis. Involvement of the abdominal aorta and the major visceral arteries is presented as concentric mural thickening, transmural calcification, luminal stenosis, occlusion, aneurysmal changes, and collateral vessels. Such findings can be observed particularly in large- and medium-vessel vasculitis. The presence of extra-intestinal findings, including within the liver, kidneys, or spleen in the form of focal areas of infarction or heterogeneous enhancement due to microvascular involvement, can be another radiologic clue in diagnosis of vasculitis. The link between the clinical/laboratory findings and MRE/CTE abnormalities needs to be corresponded when it comes to the diagnosis of intestinal vasculitis.
Keywords: Computed tomography enterography; Intestines; Magnetic resonance enterography; Vasculitis.
© 2022. The Author(s).
Conflict of interest statement
All authors have no competing interests.
Figures










References
-
- Saadoun D, Vautier M, Cacoub P. Medium- and large-vessel vasculitis. Circulation. 2021;143(3):267–282. - PubMed
-
- Broncano J, Vargas D, Bhalla S, Cummings KW, Raptis CA, Luna A. CT and MR imaging of cardiothoracic vasculitis. Radiographics. 2018;38(4):997–1021. - PubMed
-
- Konttinen YT, Rotar Z, Pettersson T, Nordstrom DC, Bacon P, Petersen J. Roadmap to vasculitis. Acta Reumatol Port. 2006;31(1):15–36. - PubMed
-
- Ha HK, Lee SH, Rha SE, et al. Radiologic features of vasculitis involving the gastrointestinal tract. Radiographics. 2000;20(3):779–794. - PubMed
-
- Zhang X, Furth EE, Tondon R. Vasculitis involving the gastrointestinal system is often incidental but critically important. Am J Clin Pathol. 2020;154(4):536–552. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

