Spasticity-related pain in children/adolescents with cerebral palsy. Part 2: IncobotulinumtoxinA efficacy results from a pooled analysis
- PMID: 36057802
- PMCID: PMC10116134
- DOI: 10.3233/PRM-220020
Spasticity-related pain in children/adolescents with cerebral palsy. Part 2: IncobotulinumtoxinA efficacy results from a pooled analysis
Abstract
Purpose: This pooled analysis of data from three Phase 3 studies investigated the effects of incobotulinumtoxinA on spasticity-related pain (SRP) in children/adolescents with uni-/bilateral cerebral palsy (CP).
Methods: Children/adolescents (ambulant and non-ambulant) were evaluated for SRP on increasingly difficult activities/tasks 4 weeks after each of four incobotulinumtoxinA injection cycles (ICs) using the Questionnaire on Pain caused by Spasticity (QPS; six modules specific to lower limb [LL] or upper limb [UL] spasticity and respondent type [child/adolescent, interviewer, or parent/caregiver]). IncobotulinumtoxinA doses were personalized, with all doses pooled for analysis.
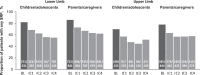
Results: QPS key item responses were available from 331 and 155 children/adolescents with LL- and UL-spasticity, respectively, and 841/444 (LL/UL) of their parents/caregivers. IncobotulinumtoxinA efficacy was evident with the first IC. Efficacy was sustained and became more robust with further subsequent ICs. By Week 4 of the last (i.e. fourth) IC, 33.8-53.3% of children/adolescents reported complete SRP relief from their baseline pain for respective QPS items. Children/adolescents reported reductions in mean LL SRP intensity at levels that surpassed clinically meaningful thresholds. Similarly, parents/caregivers observed complete SRP relief and less frequent SRP with incobotulinumtoxinA. Similar results were found for UL SRP.
Conclusion: These findings indicate that incobotulinumtoxinA could bring considerable benefit to children/adolescents with spasticity by reducing SRP, even during strenuous activities.
Keywords: Botulinum toxin; all movement disorders; all pediatric; cerebral palsy; muscle spasticity; pain.
Conflict of interest statement
Florian Heinen has received speaker’s honoraria from Allergan plc, Desitin, Ipsen Biopharmaceuticals, Merz Therapeutics, and Novartis and unrestricted educational grants from Allergan and Merz Therapeutics. Michaela Bonfert has received research grants from the HABA Foundation, the Deutsche Rentenversicherung, the Deutsche Migräne und Kopfschmerzgesellschaft, the European Research Council, ERA-NET Neuron, CSL Behring, and ZNS - Hannelore Kohl Foundation, and a research scholarship of the Bavarian Gender Equality Grant of the Free State of Bavaria, Germany. Petr Kaňovský has received speaker’s honoraria from Desitin, Ipsen Biopharmaceuticals, Merz Therapeutics, and Medtronic. A. Sebastian Schroeder has received speaker’s honoraria from and participated in advisory boards for Allergan plc, Ipsen Biopharmaceuticals, and Merz Therapeutics. Henry G. Chambers serves as a consultant for Orthopediatrics Corp and Allergan Corporation. Edward Dabrowski has participated in an advisory board and speaker bureau for Ipsen Biopharmaceuticals. Thorin L. Geister, Angelika Hanschmann, and Michael Althaus are employees of Merz Therapeutics GmbH. Marta Banach has served as a consultant and speaker and participated in an advisory board for Merz Therapeutics and has served as a speaker for Allergan, Ipsen, and Kedrion. Deborah Gaebler-Spira has served as a consultant for Teva and Kashiva.
Figures
Similar articles
-
Spasticity-related pain in children/adolescents with cerebral palsy. Part 1: Prevalence and clinical characteristics from a pooled analysis.J Pediatr Rehabil Med. 2022;15(1):129-143. doi: 10.3233/PRM-220011. J Pediatr Rehabil Med. 2022. PMID: 35342060 Free PMC article.
-
Safety and efficacy of repeat long-term incobotulinumtoxinA treatment for lower limb or combined upper/lower limb spasticity in children with cerebral palsy.J Pediatr Rehabil Med. 2022;15(1):113-127. doi: 10.3233/PRM-210041. J Pediatr Rehabil Med. 2022. PMID: 34957963 Free PMC article. Clinical Trial.
-
IncobotulinumtoxinA Efficacy/Safety in Upper-Limb Spasticity in Pediatric Cerebral Palsy: Randomized Controlled Trial.Pediatr Neurol. 2021 Oct;123:10-20. doi: 10.1016/j.pediatrneurol.2021.05.014. Epub 2021 May 21. Pediatr Neurol. 2021. PMID: 34339951 Clinical Trial.
-
Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence.J Neural Transm (Vienna). 2009 Mar;116(3):319-31. doi: 10.1007/s00702-008-0175-8. Epub 2009 Jan 14. J Neural Transm (Vienna). 2009. PMID: 19142573 Review.
-
[Treatment of spasticity in children with cerebral palsy with botulinum toxin A].Ugeskr Laeger. 2015 Jan 12;177(3):V07140409. Ugeskr Laeger. 2015. PMID: 25613095 Review. Danish.
Cited by
-
Does botulinum neurotoxin A make walking easier in children with cerebral palsy? A randomized clinical trial.Dev Med Child Neurol. 2025 Feb;67(2):263-271. doi: 10.1111/dmcn.16038. Epub 2024 Jul 26. Dev Med Child Neurol. 2025. PMID: 39058740 Free PMC article. Clinical Trial.
-
Understanding Clinical Effectiveness and Safety Implications of Botulinum Toxin in Children: A Narrative Review of the Literature.Toxins (Basel). 2024 Jul 4;16(7):306. doi: 10.3390/toxins16070306. Toxins (Basel). 2024. PMID: 39057946 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

