Exploiting colour space geometry for visual stimulus design across animals
- PMID: 36058250
- PMCID: PMC9441238
- DOI: 10.1098/rstb.2021.0280
Exploiting colour space geometry for visual stimulus design across animals
Abstract
Colour vision represents a vital aspect of perception that ultimately enables a wide variety of species to thrive in the natural world. However, unified methods for constructing chromatic visual stimuli in a laboratory setting are lacking. Here, we present stimulus design methods and an accompanying programming package to efficiently probe the colour space of any species in which the photoreceptor spectral sensitivities are known. Our hardware-agnostic approach incorporates photoreceptor models within the framework of the principle of univariance. This enables experimenters to identify the most effective way to combine multiple light sources to create desired distributions of light, and thus easily construct relevant stimuli for mapping the colour space of an organism. We include methodology to handle uncertainty of photoreceptor spectral sensitivity as well as to optimally reconstruct hyperspectral images given recent hardware advances. Our methods support broad applications in colour vision science and provide a framework for uniform stimulus designs across experimental systems. This article is part of the theme issue 'Understanding colour vision: molecular, physiological, neuronal and behavioural studies in arthropods'.
Keywords: Python; colour management; colour space; colour vision; nonlinear optimization; univariance.
Figures
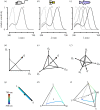
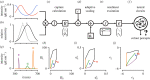
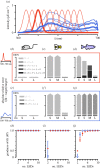


Similar articles
-
High diversity of arthropod colour vision: from genes to ecology.Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210273. doi: 10.1098/rstb.2021.0273. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058249 Free PMC article.
-
Understanding insect colour constancy.Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210286. doi: 10.1098/rstb.2021.0286. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058239 Free PMC article. Review.
-
Colour vision in nocturnal insects.Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210285. doi: 10.1098/rstb.2021.0285. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058247 Free PMC article. Review.
-
Colour vision in ants (Formicidae, Hymenoptera).Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210291. doi: 10.1098/rstb.2021.0291. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058251 Free PMC article. Review.
-
Adaptive plasticity during the development of colour vision.Prog Retin Eye Res. 2005 Jul;24(4):521-36. doi: 10.1016/j.preteyeres.2005.01.002. Prog Retin Eye Res. 2005. PMID: 15845347 Review.
Cited by
-
A chromatic feature detector in the retina signals visual context changes.Elife. 2024 Oct 4;13:e86860. doi: 10.7554/eLife.86860. Elife. 2024. PMID: 39365730 Free PMC article.
-
High diversity of arthropod colour vision: from genes to ecology.Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210273. doi: 10.1098/rstb.2021.0273. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058249 Free PMC article.
-
Hue selectivity from recurrent circuitry in Drosophila.Nat Neurosci. 2024 Jun;27(6):1137-1147. doi: 10.1038/s41593-024-01640-4. Epub 2024 May 16. Nat Neurosci. 2024. PMID: 38755272 Free PMC article.
References
-
- Stockman A, Brainard DH. 2010. Color vision mechanisms. In The OSA handbook of optics, 3rd edn (ed. M. Bass), pp. 11.1-11.104. New York, NY: McGraw-Hill.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

