Nomenclature for Cellular and Genetic Therapies: A Need for Standardization
- PMID: 36058548
- PMCID: PMC9729423
- DOI: 10.1016/j.jtct.2022.08.029
Nomenclature for Cellular and Genetic Therapies: A Need for Standardization
Abstract
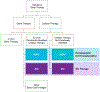
As the field of cellular and genetic therapies transitions from a scientific concept to a clinical reality, it has become evident that there are several conflicting or imprecise nomenclatures to describe these novel therapeutic products. The lack of uniformity and accuracy in the terminology often creates regulatory, educational, administrative, and billing quagmires. Standardization of the nomenclature for these therapeutic products is essential for creation of a harmonized regulatory and developmental framework, development of training paradigms and educational programs, equitable and rational decisions about accessibility, and consistency in the billing and coding structures used for reimbursement. Here we propose an updated framework as a foundation for categorizing these cell-based and genetically modified therapies.
Keywords: Cellular therapy; Gene therapy; Immune effector cell therapy.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. All rights reserved.
Figures
References
-
- National Cancer Institute. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. Vol 2022. Bethesda, MD: 2022.
-
- Grady D Cell Therapy Shows Promise for Acute Type of Leukemia. The New York Times. New York City, NY: The New York Times Company; 2013.
-
- Novartis Pharmaceuticals Corporation. KYMRIAH® (tisagenlecleucel) | Official Patient Website. Vol 20222021.
-
- US Food and Drug Administration. FDA approval brings first gene therapy to the United States. Press Announcements. Vol 2022. Silver Spring, MD: US Food and Drug Administration; 2017.
-
- Foundation for the Accreditation of Cellular Therapy, The Joint Accreditation Committee – ISCT and EBMT. Standards for Immune Effector Cells. First Edition ed: The Foundation for the Accreditation of Cellular Therapy; 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials