Optimal postoperative pain management after VATS lung resection by thoracic epidural analgesia, continuous paravertebral block or single-shot intercostal nerve block (OPtriAL): study protocol of a three-arm multicentre randomised controlled trial
- PMID: 36058900
- PMCID: PMC9441091
- DOI: 10.1186/s12893-022-01765-y
Optimal postoperative pain management after VATS lung resection by thoracic epidural analgesia, continuous paravertebral block or single-shot intercostal nerve block (OPtriAL): study protocol of a three-arm multicentre randomised controlled trial
Abstract
Background: Adequate pain control after video-assisted thoracoscopic surgery (VATS) for lung resection is important to improve postoperative mobilisation, recovery, and to prevent pulmonary complications. So far, no consensus exists on optimal postoperative pain management after VATS anatomic lung resection. Thoracic epidural analgesia (TEA) is the reference standard for postoperative pain management following VATS. Although the analgesic effect of TEA is clear, it is associated with patient immobilisation, bladder dysfunction and hypotension which may result in delayed recovery and longer hospitalisation. These disadvantages of TEA initiated the development of unilateral regional techniques for pain management. The most frequently used techniques are continuous paravertebral block (PVB) and single-shot intercostal nerve block (ICNB). We hypothesize that using either PVB or ICNB is non-inferior to TEA regarding postoperative pain and superior regarding quality of recovery (QoR). Signifying faster postoperative mobilisation, reduced morbidity and shorter hospitalisation, these techniques may therefore reduce health care costs and improve patient satisfaction.
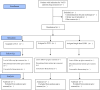
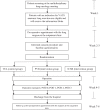
Methods: This multi-centre randomised study is a three-arm clinical trial comparing PVB, ICNB and TEA in a 1:1:1 ratio for pain (non-inferiority) and QoR (superiority) in 450 adult patients undergoing VATS anatomic lung resection. Patients will not be eligible for inclusion in case of contraindications for TEA, PVB or ICNB, chronic opioid use or if the lung surgeon estimates a high probability that the operation will be performed by thoracotomy.
Primary outcomes: (1) the proportion of pain scores ≥ 4 as assessed by the numerical rating scale (NRS) measured during postoperative days (POD) 0-2; and (2) the QoR measured with the QoR-15 questionnaire on POD 1 and 2. Secondary outcome measures are cumulative use of opioids and analgesics, postoperative complications, hospitalisation, patient satisfaction and degree of mobility.
Discussion: The results of this trial will impact international guidelines with respect to perioperative care optimization after anatomic lung resection performed through VATS, and will determine the most cost-effective pain strategy and may reduce variability in postoperative pain management. Trial registration The trial is registered at the Netherlands Trial Register (NTR) on February 1st, 2021 (NL9243). The NTR is no longer available since June 24th, 2022 and therefore a revised protocol has been registered at ClinicalTrials.gov on August 5th, 2022 (NCT05491239).
Protocol version: version 3 (date 06-05-2022), ethical approval through an amendment (see ethical proof in the Study protocol proof).
Keywords: ERATS; Intercostal nerve block; Locoregional anaesthesia; Paravertebral block; Postoperative pain; Thoracic epidural; VATS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto. General Hospital Transitional Pain Service General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49–58. doi: 10.1080/24740527.2019.1574537. - DOI - PMC - PubMed
-
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERASVR) Society and the European Society of Thoracic Surgeons (ESTS) Eur J Cardio-Thoracic Surg. 2019;55:91–115. doi: 10.1093/ejcts/ezy301. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical