Protein O-GlcNAcylation and the regulation of energy homeostasis: lessons from knock-out mouse models
- PMID: 36058931
- PMCID: PMC9443036
- DOI: 10.1186/s12929-022-00851-w
Protein O-GlcNAcylation and the regulation of energy homeostasis: lessons from knock-out mouse models
Abstract
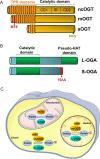
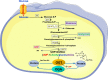
O-GlcNAcylation corresponds to the addition of N-Acetylglucosamine (GlcNAc) on serine or threonine residues of cytosolic, nuclear and mitochondrial proteins. This reversible modification is catalysed by a unique couple of enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). OGT uses UDP-GlcNAc produced in the hexosamine biosynthesis pathway, to modify proteins. UDP-GlcNAc is at the cross-roads of several cellular metabolisms, including glucose, amino acids and fatty acids. Therefore, OGT is considered as a metabolic sensor that post-translationally modifies proteins according to nutrient availability. O-GlcNAcylation can modulate protein-protein interactions and regulate protein enzymatic activities, stability or subcellular localization. In addition, it can compete with phosphorylation on the same serine or threonine residues, or regulate positively or negatively the phosphorylation of adjacent residues. As such, O-GlcNAcylation is a major actor in the regulation of cell signaling and has been implicated in numerous physiological and pathological processes. A large body of evidence have indicated that increased O-GlcNAcylation participates in the deleterious effects of glucose (glucotoxicity) in metabolic diseases. However, recent studies using mice models with OGT or OGA knock-out in different tissues have shown that O-GlcNAcylation protects against various cellular stresses, and indicate that both increase and decrease in O-GlcNAcylation have deleterious effects on the regulation of energy homeostasis.
Keywords: Glucotoxicity; Inflammation; Knock-out mouse models; Metabolic diseases; O-GlcNAc transferase; O-GlcNAcase; O-GlcNAcylation; Oxidative stress.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

