Brain tumor related epilepsy: pathophysiological approaches and rational management of antiseizure medication
- PMID: 36059029
- PMCID: PMC9442934
- DOI: 10.1186/s42466-022-00205-9
Brain tumor related epilepsy: pathophysiological approaches and rational management of antiseizure medication
Abstract
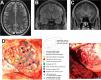
Background: Brain tumor related epilepsy (BTRE) is a common complication of cerebral tumors and its incidence is highly dependent on the type of tumor, ranging from 10-15% in brain metastases to > 80% in low grade gliomas. Clinical management is challenging and has to take into account aspects beyond the treatment of non-tumoral epilepsy.
Main body: Increasing knowledge about the pathophysiology of BTRE, particularly on glutamatergic mechanisms of oncogenesis and epileptogenesis, might influence management of anti-tumor and BTRE treatment in the future. The first seizure implies the diagnosis of epilepsy in patients with brain tumors. Due to the lack of prospective randomized trials in BTRE, general recommendations for focal epilepsies currently apply concerning the initiation of antiseizure medication (ASM). Non-enzyme inducing ASM is preferable. Prospective trials are needed to evaluate, if AMPA inhibitors like perampanel possess anti-tumor effects. ASM withdrawal has to be weighed very carefully against the risk of seizure recurrence, but can be achievable in selected patients. Permission to drive is possible for some patients with BTRE under well-defined conditions, but requires thorough neurological, radiological, ophthalmological and neuropsychological examination.
Conclusion: An evolving knowledge on pathophysiology of BTRE might influence future therapy. Randomized trials on ASM in BTRE with reliable endpoints are needed. Management of withdrawal of ASMs and permission to drive demands thorough diagnostic as well as neurooncological and epileptological expertise.
Keywords: Antiseizure medication; Brain metastasis; Brain tumor related epilepsy; Glioma.
© 2022. The Author(s).
Conflict of interest statement
T.W. has received of honoraria or consultation fees Eisai, UCB, GW, Angelini, Precisis and is a stock shareholder of Amgen. J.W. has received speaker fees from UCB, Eisai, Bial, GW pharmaceuticals, and Desitin. U.S. received speaker’s honoraria from Medac, GSK and Novartis. W.G. has received speaker´s honoraria, travel or accommodation payment fees from UCB, Eisei, Bial and Desitin. The other authors declare no competing interests.
Figures
References
-
- le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, Galldiks N, de Azambuja E, Berghoff AS, Metellus P, Peters S, Hong Y-K, Winkler F, Schadendorf D, van den Bent M, Seoane J, Stahel R, Minniti G, Wesseling P, Weller M, Preusser M. EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Annals of Oncology. 2021;32(11):1332–1347. doi: 10.1016/j.annonc.2021.07.016. - DOI - PubMed
-
- Pallud J, Audureau E, Blonski M, Sanai N, Bauchet L, Fontaine D, Mandonnet E, Dezamis E, Psimaras D, Guyotat J, Peruzzi P, Page P, Gal B, Párraga E, Baron M-H, Vlaicu M, Guillevin R, Devaux B, Duffau H, Taillandier L, Capelle L, Huberfeld G. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(2):449–462. doi: 10.1093/brain/awt345. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
