Modulation of dendritic cell metabolism by an MPLA-adjuvanted allergen product for specific immunotherapy
- PMID: 36059475
- PMCID: PMC9430023
- DOI: 10.3389/fimmu.2022.916491
Modulation of dendritic cell metabolism by an MPLA-adjuvanted allergen product for specific immunotherapy
Abstract
Background: Recently, bacterial components were shown to enhance immune responses by shifting immune cell metabolism towards glycolysis and lactic acid production, also known as the Warburg Effect. Currently, the effect of allergen products for immunotherapy (AIT) and commercial vaccines on immune cell metabolism is mostly unknown.
Objective: To investigate the effect of AIT products (adjuvanted with either MPLA or Alum) on myeloid dendritic cell (mDC) metabolism and activation.
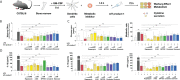
Methods: Bone marrow-derived mDCs were stimulated with five allergoid-based AIT products (one adjuvanted with MPLA, four adjuvanted with Alum) and two MPLA-adjuvanted vaccines and analyzed for their metabolic activation, expression of cell surface markers, and cytokine secretion by ELISA. mDCs were pre-incubated with either immunological or metabolic inhibitors or cultured in glucose- or glutamine-free culture media and subsequently stimulated with the MPLA-containing AIT product (AIT product 1). mDCs were co-cultured with allergen-specific CD4+ T cells to investigate the contribution of metabolic pathways to the T cell priming capacity of mDCs stimulated with AIT product 1.
Results: Both the MPLA-containing AIT product 1 and commercial vaccines, but not the Alum-adjuvanted AIT products, activated Warburg metabolism and TNF-α secretion in mDCs. Further experiments focused on AIT product 1. Metabolic analysis showed that AIT product 1 increased glycolytic activity while also inducing the secretion of IL-1β, IL-10, IL-12, and TNF-α. Both rapamycin (mTOR-inhibitor) and SP600125 (SAP/JNK MAPK-inhibitor) dose-dependently suppressed the AIT product 1-induced Warburg Effect, glucose consumption, IL-10-, and TNF-α secretion. Moreover, both glucose- and glutamine deficiency suppressed secretion of all investigated cytokines (IL-1β, IL-10, and TNF-α). Glucose metabolism in mDCs was also critical for the (Th1-biased) T cell priming capacity of AIT product 1-stimulated mDCs, as inhibition of mTOR signaling abrogated their ability to induce Th1-responses.
Conclusion: The AIT product and commercial vaccines containing the adjuvant MPLA were shown to modulate the induction of immune responses by changing the metabolic state of mDCs. Better understanding the mechanisms underlying the interactions between cell metabolism and immune responses will allow us to further improve vaccine development and AIT.
Keywords: MPLA: monophosphoryl lipid A; Warburg Effect; allergen specific immunotherapy; immune metabolism; vaccine.
Copyright © 2022 Zimmermann, Goretzki, Meier, Wolfheimer, Lin, Rainer, Krause, Wedel, Spies, Führer, Vieths, Scheurer and Schülke.
Conflict of interest statement
The authors are employees of the German Federal Institute for Vaccines and Biomedicines. The Paul-Ehrlich-Institut (PEI) is an Agency of the German Federal Ministry of Health. In relation to the present publication, the authors consider themselves not having a conflict of interest. Opinions expressed in the paper are personal views of the authors, not necessarily reflecting an official opinion of the PEI or the German Federal Ministry of Health.
Figures







References
-
- Schülke S, Fiedler A-H, Ann-Christine J, Flaczyk A, Wolfheimer S, Anke H, et al. . Critical role of mammalian target of rapamycin for IL-10 DC induction by a flagellin FlaA-conjugate preventing allergic sensitization. J Allergy Clin Immunol (2018) 141(5):1786–98. doi: 10.3390/cells101026140.1016/j.jaci.2017.07.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

