Methamphetamine induces transcriptional changes in cultured HIV-infected mature monocytes that may contribute to HIV neuropathogenesis
- PMID: 36059515
- PMCID: PMC9433802
- DOI: 10.3389/fimmu.2022.952183
Methamphetamine induces transcriptional changes in cultured HIV-infected mature monocytes that may contribute to HIV neuropathogenesis
Abstract
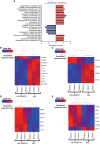
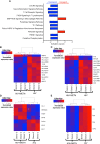
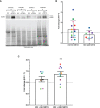
HIV-associated neurocognitive impairment (HIV-NCI) persists in 15-40% of people with HIV (PWH) despite effective antiretroviral therapy. HIV-NCI significantly impacts quality of life, and there is currently no effective treatment for it. The development of HIV-NCI is complex and is mediated, in part, by the entry of HIV-infected mature monocytes into the central nervous system (CNS). Once in the CNS, these cells release inflammatory mediators that lead to neuroinflammation, and subsequent neuronal damage. Infected monocytes may infect other CNS cells as well as differentiate into macrophages, thus contributing to viral reservoirs and chronic neuroinflammation. Substance use disorders in PWH, including the use of methamphetamine (meth), can exacerbate HIV neuropathogenesis. We characterized the effects of meth on the transcriptional profile of HIV-infected mature monocytes using RNA-sequencing. We found that meth mediated an upregulation of gene transcripts related to viral infection, cell adhesion, cytoskeletal arrangement, and extracellular matrix remodeling. We also identified downregulation of several gene transcripts involved in pathogen recognition, antigen presentation, and oxidative phosphorylation pathways. These transcriptomic changes suggest that meth increases the infiltration of mature monocytes that have a migratory phenotype into the CNS, contributing to dysregulated inflammatory responses and viral reservoir establishment and persistence, both of which contribute to neuronal damage. Overall, our results highlight potential molecules that may be targeted for therapy to limit the effects of meth on HIV neuropathogenesis.
Keywords: HIV; methamphetamine; migration; monocytes; neuroinflammation; viral reservoirs.
Copyright © 2022 Chilunda, Weiselberg, Martinez-Meza, Mhamilawa, Cheney and Berman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- UNAIDS . Global HIV & AIDS statistics — fact sheet 2022 [cited 2022 march 27th]. Available at: https://www.unaids.org/en/resources/fact-sheet
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

