Cross-sectional analysis of the humoral response after SARS-CoV-2 vaccination in Sardinian multiple sclerosis patients, a follow-up study
- PMID: 36059537
- PMCID: PMC9433902
- DOI: 10.3389/fimmu.2022.946356
Cross-sectional analysis of the humoral response after SARS-CoV-2 vaccination in Sardinian multiple sclerosis patients, a follow-up study
Abstract
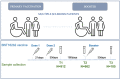
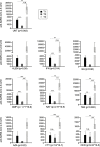
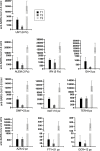
Monitoring immune responses to SARS-CoV-2 vaccination and its clinical efficacy over time in Multiple Sclerosis (MS) patients treated with disease-modifying therapies (DMTs) help to establish the optimal strategies to ensure adequate COVID-19 protection without compromising disease control offered by DMTs. Following our previous observations on the humoral response one month after two doses of BNT162b2 vaccine (T1) in MS patients differently treated, here we present a cross-sectional and longitudinal follow-up analysis six months following vaccination (T2, n=662) and one month following the first booster (T3, n=185). Consistent with results at T1, humoral responses were decreased in MS patients treated with fingolimod and anti-CD20 therapies compared with untreated patients also at the time points considered here (T2 and T3). Interestingly, a strong upregulation one month after the booster was observed in patients under every DMTs analyzed, including those treated with fingolimod and anti-CD20 therapies. Although patients taking these latter therapies had a higher rate of COVID-19 infection five months after the first booster, only mild symptoms that did not require hospitalization were reported for all the DMTs analyzed here. Based on these findings we anticipate that additional vaccine booster shots will likely further improve immune responses and COVID-19 protection in MS patients treated with any DMT.
Keywords: BNT162b2 messenger RNA (mRNA); COVID-19; COVID-19 vaccination; SARS-CoV-2; disease-modifying therapy (DMT); humoral immunity; multiple sclerosis; vaccine efficacy and effectiveness.
Copyright © 2022 Idda, Pitzalis, Lodde, Loizedda, Frau, Lobina, Zoledziewska, Virdis, Delogu, Marini, Mingoia, Masala, Lorefice, Fronza, Carmagnini, Carta, Pilotto, Castiglia, Chessa, Uzzau, Farina, Solla, Steri, Devoto, Fiorillo, Floris, Zarbo, Cocco and Cucca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

