Comparative review of pharmacological therapies in individuals with HER2-positive advanced breast cancer with focus on hormone receptor subgroups
- PMID: 36059633
- PMCID: PMC9433866
- DOI: 10.3389/fonc.2022.943154
Comparative review of pharmacological therapies in individuals with HER2-positive advanced breast cancer with focus on hormone receptor subgroups
Abstract
Breast cancer is the fifth leading cause of cancer-related deaths worldwide. The randomized controlled trials (RCTs) of targeted therapies in human epidermal receptor 2 (HER2)-positive advanced breast cancer (ABC) have provided an evidence base for regulatory and reimbursement agencies to appraise the use of cancer therapies in clinical practice. However, a subset of these patients harbor additional biomarkers, for example, a positive hormone receptor status that may be more amenable to therapy and improve overall survival (OS). This review seeks to explore the reporting of evidence for treatment effects by the hormone receptor status using the RCT evidence of targeted therapies for HER2-positive ABC patients. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed to identify published RCTs. Extracted data were synthesized using network meta-analysis to obtain the relative effects of HER2-positive-targeted therapies. We identified a gap in the reporting of the effectiveness of therapies by the hormone receptor status as only 15 out of 42 identified RCTs reported hormone receptor subgroup analyses; the majority of which reported progression-free survival but not OS or the overall response rate. In conclusion, we recommend that future trials in ABC should report the effect of cancer therapies in hormone receptor subgroups for all outcomes.
Keywords: Advanced breast cancer; HER2 positive; hormone receptor; metastatic breast cancer; network meta-analysis; subgroup analysis; targeted therapies.
Copyright © 2022 Umemneku-Chikere, Ayodele, Soares, Khan, Abrams, Owen and Bujkiewicz.
Conflict of interest statement
SB is a member of the NICE Decision Support Unit. She served as a paid consultant, providing unrelated methodological advice to the NICE, pharmaceutical industry and consultancy companies. She received payments for educational events from Roche and has received research funding from European Federation of Pharmaceutical Industries and Association (EFPAI) and Johnson and Johnson. RO is a member of the National Institute for health and Care Excellence (NICE) Technology Appraisal committee, member of the NICE Decision Support Unit (DSU), and associate member of the NICE Technical Support Unit (TSU). She has served as a paid consultant to the pharmaceutical industry, providing unrelated methodological advice. She reports teaching fees from the Association of British Pharmaceutical Industry (ABPI) and the University of Bristol. KA is a member of the National Institute for Health and Care Excellence (NICE) Diagnostics Advisory Committee and is a National Institute for Health and Care Research (NIHR) Senior Investigator Emeritus. He has acted as a paid consultant, providing unrelated methodological and strategic advice, to the pharmaceutical and life sciences industry generally, as well as to UK Department of Health and Social Care (DHSC)/NICE, and has received unrelated research funding from; Association of the British Pharmaceutical Industry (ABPI), European Federation of Pharmaceutical Industries and Associations (EFPIA), Pfizer, Sanofi and Swiss Precision Diagnostics. He has also received course fees from ABPI and is a Partner/Director of Visible Analytics Limited. SK is supported by a NIHR academic Clinical Lecturer award and has no conflicts of interest to declare. MS is a member of a research funding panel for the National Institute for Health and Care Research (NIHR), and collaborates with the NICE Decision Support Unit (DSU). She has served as a paid consultant to the pharmaceutical and life sciences industry generally, as well as to DHSC/NICE, providing unrelated methodological advice. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
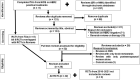
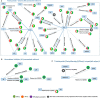




References
-
- Park Y, Senkus-Konefka E, Im S-A, Pentheroudakis G, Saji S, Gupta S, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with early breast cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol (2020) 31(4):451–69. doi: 10.1016/j.annonc.2020.01.008 - DOI - PubMed
-
- Bai X, Lin X, Song J, Chang JH, Han LL, Fan C. Incidence of central nervous system metastases in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer treated with trastuzumab: A meta-analysis. Clinics (Sao Paulo) (2021) 76:e2653. doi: 10.6061/clinics/2021/e2653 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

