Metabolic management of microenvironment acidity in glioblastoma
- PMID: 36059707
- PMCID: PMC9428719
- DOI: 10.3389/fonc.2022.968351
Metabolic management of microenvironment acidity in glioblastoma
Abstract
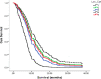
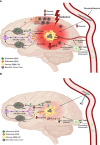
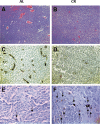
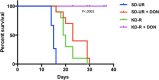
Glioblastoma (GBM), similar to most cancers, is dependent on fermentation metabolism for the synthesis of biomass and energy (ATP) regardless of the cellular or genetic heterogeneity seen within the tumor. The transition from respiration to fermentation arises from the documented defects in the number, the structure, and the function of mitochondria and mitochondrial-associated membranes in GBM tissue. Glucose and glutamine are the major fermentable fuels that drive GBM growth. The major waste products of GBM cell fermentation (lactic acid, glutamic acid, and succinic acid) will acidify the microenvironment and are largely responsible for drug resistance, enhanced invasion, immunosuppression, and metastasis. Besides surgical debulking, therapies used for GBM management (radiation, chemotherapy, and steroids) enhance microenvironment acidification and, although often providing a time-limited disease control, will thus favor tumor recurrence and complications. The simultaneous restriction of glucose and glutamine, while elevating non-fermentable, anti-inflammatory ketone bodies, can help restore the pH balance of the microenvironment while, at the same time, providing a non-toxic therapeutic strategy for killing most of the neoplastic cells.
Keywords: fermentation; glutamate; glutaminolysis; glycolysis; ketogenic diet; ketogenic metabolic therapy; lactate; succinate.
Copyright © 2022 Seyfried, Arismendi-Morillo, Zuccoli, Lee, Duraj, Elsakka, Maroon, Mukherjee, Ta, Shelton, D’Agostino, Kiebish and Chinopoulos.
Conflict of interest statement
Author KM was employed by the company BERG LLC. Author SL was employed by the company Matterworks. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as the potential conflict of interest.
Figures


References
-
- Fabbro-Peray P, Zouaoui S, Darlix A, Fabbro M, Pallud J, Rigau V, et al. . Association of patterns of care, prognostic factors, and use of radiotherapy-temozolomide therapy with survival in patients with newly diagnosed glioblastoma: a French national population-based study. J Neurooncol (2018) 142(1):91–101. doi: 10.1007/s11060-018-03065-z - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

