Podocyte-specific deletion of miR-146a increases podocyte injury and diabetic kidney disease
- PMID: 36059820
- PMCID: PMC9433550
- DOI: 10.3389/fmed.2022.897188
Podocyte-specific deletion of miR-146a increases podocyte injury and diabetic kidney disease
Abstract
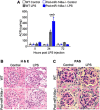
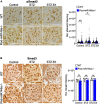
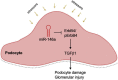
Diabetic glomerular injury is a major complication of diabetes mellitus and is the leading cause of end stage renal disease (ESRD). Healthy podocytes are essential for glomerular function and health. Injury or loss of these cells results in increased proteinuria and kidney dysfunction and is a common finding in various glomerulopathies. Thus, mechanistic understanding of pathways that protect podocytes from damage are essential for development of future therapeutics. MicroRNA-146a (miR-146a) is a negative regulator of inflammation and is highly expressed in myeloid cells and podocytes. We previously reported that miR-146a levels are significantly reduced in the glomeruli of patients with diabetic nephropathy (DN). Here we report generation of mice with selective deletion of miR-146a in podocytes and use of these mice in models of glomerular injury. Induction of glomerular injury in C57BL/6 wildtype mice (WT) and podocyte-specific miR-146a knockout (Pod-miR146a-/-) animals via administration of low-dose lipopolysaccharide (LPS) or nephrotoxic serum (NTS) resulted in increased proteinuria in the knockout mice, suggesting that podocyte-expressed miR-146a protects these cells, and thus glomeruli, from damage. Furthermore, induction of hyperglycemia using streptozotocin (STZ) also resulted in an accelerated development of glomerulopathy and a rapid increase in proteinuria in the knockout animals, as compared to the WT animals, further confirming the protective role of podocyte-expressed miR-146a. We also confirmed that the direct miR-146a target, ErbB4, was significantly upregulated in the diseased glomeruli and erlotinib, an ErbB4 and EGFR inhibitor, reducedits upregulation and the proteinuria in treated animals. Primary miR146-/- podocytes from these animals also showed a basally upregulated TGFβ-Smad3 signaling in vitro. Taken together, this study shows that podocyte-specific miR-146a is imperative for protecting podocytes from glomerular damage, via modulation of ErbB4/EGFR, TGFβ, and linked downstream signaling.
Keywords: MicroRNA; diabetic nephropathy; glomerular disease; miR-146a; podocytes.
Copyright © 2022 Li, Venkatesh, Villanueva, Wei, Geraghty, Rajagopalan, Helmuth, Altintas, Faridi and Gupta.
Conflict of interest statement
VG was an inventor on patent applications related to these studies. AR recently completed her post-doctoral fellowship and was employed by Genentech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Centers for Disease Control and Prevention. National diabetes statistics report website. Atlanta, GA: Centers for Disease Control and Prevention; (2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

