Novel Phenobarbital-Loaded Nanostructured Lipid Carriers for Epilepsy Treatment: From QbD to In Vivo Evaluation
- PMID: 36059881
- PMCID: PMC9428247
- DOI: 10.3389/fchem.2022.908386
Novel Phenobarbital-Loaded Nanostructured Lipid Carriers for Epilepsy Treatment: From QbD to In Vivo Evaluation
Abstract
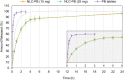
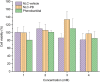
Pharmacological treatments of central nervous system diseases are always challenging due to the restrictions imposed by the blood-brain barrier: while some drugs can effectively cross it, many others, some antiepileptic drugs among them, display permeability issues to reach the site of action and exert their pharmacological effects. The development of last-generation therapeutic nanosystems capable of enhancing drug biodistribution has gained ground in the past few years. Lipid-based nanoparticles are promising systems aimed to improve or facilitate the passage of drugs through biological barriers, which have demonstrated their effectiveness in various therapeutic fields, without signs of associated toxicity. In the present work, nanostructured lipid carriers (NLCs) containing the antiepileptic drug phenobarbital were designed and optimized by a quality by design approach (QbD). The optimized formulation was characterized by its entrapment efficiency, particle size, polydispersity index, and Z potential. Thermal properties were analyzed by DSC and TGA, and morphology and crystal properties were analyzed by AFM, TEM, and XRD. Drug localization and possible interactions between the drug and the formulation components were evaluated using FTIR. In vitro release kinetic, cytotoxicity on non-tumoral mouse fibroblasts L929, and in vivo anticonvulsant activity in an animal model of acute seizures were studied as well. The optimized formulation resulted in spherical particles with a mean size of ca. 178 nm and 98.2% of entrapment efficiency, physically stable for more than a month. Results obtained from the physicochemical and in vitro release characterization suggested that the drug was incorporated into the lipid matrix losing its crystalline structure after the synthesis process and was then released following a slower kinetic in comparison with the conventional immediate-release formulation. The NLC was non-toxic against the selected cell line and capable of delivering the drug to the site of action in an adequate amount and time for therapeutic effects, with no appreciable neurotoxicity. Therefore, the developed system represents a promising alternative for the treatment of one of the most prevalent neurological diseases, epilepsy.
Keywords: PTZ test; anticonvulsant; drug delivery; epilepsy; nanostructured lipid carrier (NLC); phenobarbital; release kinetic; solid lipid nanoparticles (SLNs).
Copyright © 2022 Scioli-Montoto, Sbaraglini, Cisneros, Chain, Ferretti, León, Alvarez, Castro, Islan, Talevi and Ruiz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Andrade L. N., Marques C., Barbosa T., Santos R., Chaud M. V., da Silva C. F., et al. (2020). Praziquantel-loaded Solid Lipid Nanoparticles: Production, Physicochemical Characterization, Release Profile, Cytotoxicity and In Vitro Activity against Schistosoma Mansoni. J. Drug Deliv. Sci. Technol. 58, 101784. 10.1016/J.JDDST.2020.101784 - DOI
-
- Baker R. W., Lonsdale H. K. (1975). “Controlled Release: Mechanisms and Rates,” in Controlled Release of Biologically Active Agents. Editors Tanquary A. C., Lacey R. E. (New York: Plenum Press; ), 15–72.
LinkOut - more resources
Full Text Sources
Miscellaneous

