Generic boundaries in the Ophiostomatales reconsidered and revised
- PMID: 36059894
- PMCID: PMC9365045
- DOI: 10.3114/sim.2022.101.02
Generic boundaries in the Ophiostomatales reconsidered and revised
Abstract
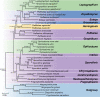
The Ophiostomatales was erected in 1980. Since that time, several of the genera have been redefined and others have been described. There are currently 14 accepted genera in the Order. They include species that are the causal agents of plant and human diseases and common associates of insects such as bark beetles. Well known examples include the Dutch elm disease fungi and the causal agents of sporotrichosis in humans and animals. The taxonomy of the Ophiostomatales was confused for many years, mainly due to the convergent evolution of morphological characters used to delimit unrelated fungal taxa. The emergence of DNA-based methods has resolved much of this confusion. However, the delineation of some genera and the placement of various species and smaller lineages remains inconclusive. In this study we reconsidered the generic boundaries within the Ophiostomatales. A phylogenomic framework constructed from genome-wide sequence data for 31 species representing the major genera in the Order was used as a guide to delineate genera. This framework also informed our choice of the best markers from the currently most commonly used gene regions for taxonomic studies of these fungi. DNA was amplified and sequenced for more than 200 species, representing all lineages in the Order. We constructed phylogenetic trees based on the different gene regions and assembled a concatenated data set utilising a suite of phylogenetic analyses. The results supported and confirmed the delineation of nine of the 14 currently accepted genera, i.e. Aureovirgo, Ceratocystiopsis, Esteya, Fragosphaeria, Graphilbum, Hawksworthiomyces, Ophiostoma, Raffaelea and Sporothrix. The two most recently described genera, Chrysosphaeria and Intubia, were not included in the multi-locus analyses. This was due to their high sequence divergence, which was shown to result in ambiguous taxonomic placement, even though the results of phylogenomic analysis supported their inclusion in the Ophiostomatales. In addition to the currently accepted genera in the Ophiostomatales, well-supported lineages emerged that were distinct from those genera. These are described as novel genera. Two lineages included the type species of Grosmannia and Dryadomyces and these genera are thus reinstated and their circumscriptions redefined. The descriptions of all genera in the Ophiostomatales were standardised and refined where this was required and 39 new combinations have been provided for species in the newly emerging genera and one new combination has been provided for Sporothrix. The placement of Afroraffaelea could not be confirmed using the available data and the genus has been treated as incertae sedis in the Ophiostomatales. Paleoambrosia was not included in this study, due to the absence of living material available for this monotypic fossil genus. Overall, this study has provided the most comprehensive and robust phylogenies currently possible for the Ophiostomatales. It has also clarified several unresolved One Fungus-One Name nomenclatural issues relevant to the Order. Taxonomic novelties: New genera: Harringtonia Z.W. de Beer & M. Procter, Heinzbutinia Z.W. de Beer & M. Procter, Jamesreidia Z.W. de Beer & M. Procter, Masuyamyces Z.W. de Beer & M. Procter. New species: Masuyamyces massonianae M. Procter & Z.W. de Beer. New combinations: Dryadomyces montetyi (M. Morelet) M. Procter & Z.W. de Beer, Dryadomyces quercivorus (Kubono & Shin. Ito) M. Procter & Z.W. de Beer, Dryadomyces quercus-mongolicae (K.H. Kim et al.) M. Procter & Z.W. de Beer, Dryadomyces sulphureus (L.R. Batra) M. Procter & Z.W. de Beer, Graphilbum pusillum (Masuya) M. Procter & Z.W. de Beer, Grosmannia abieticolens (K. Jacobs & M.J. Wingf.) M. Procter & Z.W. de Beer, Grosmannia altior (Paciura et al.) M. Procter & Z.W. de Beer, Grosmannia betulae (Jankowiak et al.) M. Procter & Z.W. de Beer, Grosmannia curviconidia (Paciura et al.) M. Procter & Z.W. de Beer, Grosmannia euphyes (K. Jacobs & M.J. Wingf.) M. Procter & Z.W. de Beer, Grosmannia fenglinhensis (R. Chang et al.) M. Procter & Z.W. de Beer, Grosmannia gestamen (de Errasti & Z.W. de Beer) M. Procter & Z.W. de Beer, Grosmannia innermongolica (X.W. Liu et al.) M. Procter & Z.W. de Beer, Grosmannia pistaciae (Paciura et al.) M. Procter & Z.W. de Beer, Grosmannia pruni (Masuya & M.J. Wingf.) M. Procter & Z.W. de Beer, Grosmannia taigensis (Linnak. et al.) M. Procter & Z.W. de Beer, Grosmannia trypodendri (Jankowiak et al.) M. Procter & Z.W. de Beer, Harringtonia aguacate (D.R. Simmons et al.) M. Procter & Z.W. de Beer, Harringtonia brunnea (L.R. Batra) M. Procter & Z.W. de Beer, Harringtonia lauricola (T.C. Harr. et al.) Z.W. de Beer & M. Procter, Heinzbutinia grandicarpa (Kowalski & Butin) Z.W. de Beer & M. Procter, Heinzbutinia microspora (Arx) M. Procter & Z.W. de Beer, Heinzbutinia solheimii (B. Strzałka & Jankowiak) Z.W. de Beer & M. Procter, Jamesreidia coronata (Olchow. & J. Reid) M. Procter & Z.W. de Beer, Jamesreidia nigricarpa (R.W. Davidson) M. Procter & Z.W. de Beer, Jamesreidia rostrocoronata (R.W. Davidson & Eslyn) M. Procter & Z.W. de Beer, Jamesreidia tenella (R.W. Davidson) Z.W. de Beer & M. Procter, Leptographium cainii (Olchow. & J. Reid) M. Procter & Z.W. de Beer, Leptographium europioides (E.F. Wright & Cain) M. Procter & Z.W. de Beer, Leptographium galeiforme (B.K. Bakshi) M. Procter & Z.W. de Beer, Leptographium pseudoeurophioides (Olchow. & J. Reid) M. Procter & Z.W. de Beer, Leptographium radiaticola (J.J. Kim et al.) M. Procter & Z.W. de Beer, Masuyamyces acarorum (R. Chang & Z.W. de Beer) M. Procter & Z.W. de Beer, Masuyamyces ambrosius (B.K. Bakshi) M. Procter & Z.W. de Beer, Masuyamyces botuliformis (Masuya) Z.W. de Beer & M. Procter, Masuyamyces jilinensis (R. Chang et al.) M. Procter & Z.W. de Beer, Masuyamyces lotiformis (Z. Wang & Q. Lu) M. Procter & Z.W. de Beer, Masuyamyces pallidulus (Linnak. et al.) M. Procter & Z.W. de Beer, Masuyamyces saponiodorus (Linnak. et al.) M. Procter & Z.W. de Beer, Sporothrix longicollis (Massee & E.S. Salmon) M. Procter & Z.W. de Beer. Citation: de Beer W, Procter M, Wingfield MJ, Marincowitz S, Duong TA (2022). Generic boundaries in the Ophiostomatales reconsidered and revised. Studies in Mycology 101: 57-120. doi: 10.3114/sim.2022.101.02.
Keywords: Generic boundaries; Ophiostomataceae; Ophiostomatales; Sordariomycetidae; new taxa; nomenclature; taxonomy.
© 2022 Westerdijk Fungal Biodiversity Institute.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures














References
-
- Aghayeva DN, Wingfield MJ, De Beer ZW, et al. (2004). Two new Ophiostoma species with Sporothrix anamorphs from Austria and Azerbaijan. Mycologia 96: 866–878. - PubMed
-
- Ando Y, Masuya H, Motohashi K, et al. (2016). Phylogenetic relationship of Japanese isolates belonging to the Grosmannia piceiperda complex (Ophiostomatales). Mycoscience 57: 123–135.
-
- Bakshi BK. (1950). Fungi associated with ambrosia beetles in Great Britain. Transactions of the British Mycological Society 33: 111–120.
-
- Bakshi BK. (1951). Studies on four species of Ceratocystis, with a discussion on fungi causing sap-stain in Britain. Mycological Paper 35: 1–16.
-
- Bateman C, Huang Y-T, Simmons DR, et al. (2017). Ambrosia beetle Premnobius cavipennis (Scolytinae: Ipini) carries highly divergent ascomycotan ambrosia fungus, Afroraffaelea ambrosiae gen. nov. et sp. nov. (Ophiostomatales). Fungal Ecology 25: 41–49.
LinkOut - more resources
Full Text Sources
Miscellaneous
