Therapeutic effects of shaogan fuzi decoction in rheumatoid arthritis: Network pharmacology and experimental validation
- PMID: 36059943
- PMCID: PMC9428562
- DOI: 10.3389/fphar.2022.967164
Therapeutic effects of shaogan fuzi decoction in rheumatoid arthritis: Network pharmacology and experimental validation
Abstract
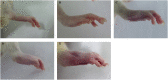
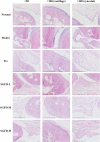
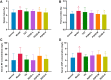
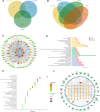
Shaogan Fuzi Decoction (SGFD), one of the classical prescriptions of Chinese Medicine, has a long history in the treatment of rheumatoid arthritis (RA), but definitive studies on its efficacy and mechanism of action are lacking. This study aims to elucidate the pharmacodynamic role of SGFD against RA and the potential mechanisms based on a combination of network pharmacology and experimental verification. The RA model in rats was induced by intradermal injection of bovine type Ⅱ collagen and incomplete Freund's adjuvant at the tail root. SGFD was administered once a day by oral gavage for 4 weeks. After SGFD administration, rat's arthritis index (AI) score and paw swelling decreased to some extent, and synovial inflammation, vascular hyperplasia, and cartilage destruction of the ankle joint were improved. Simultaneously, thymus and spleen index and serum levels of C-reactive protein (CRP) were lowered. Network pharmacology revealed that quercetin, kaempferol, naringenin, formononetin isorhamnetin and licochalcone A were the potentialiy active components, and IL6, TP53, TNF, PTGS2, MAPK3 and IL-1β were potential key targets for SGFD in the treatment of RA. Ingredients-targets molecular docking showed that the components had the high binding activity to these target proteins. The mechanism of SGFD for RA involves various biological functions and is closely correlated with TNF signaling pathway, Osteoclast differentiation, T cell receptor signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, NF-κB signaling pathway, toll-like receptor signaling pathway, and so on. Western blot and ELISA showed that the expression of toll-like receptor 4 (TLR4), nuclear factor kappa-B (NF-κB) p65, phosphorylated c-Jun N-terminal kinase (p-JNK), p-p38, phosphorylated extracellular regulated kinase (p-ERK) and TNF-α was significantly upregulated in the synovium of RA rats, and the levels of serum inflammatory factors were significantly increased. SGFD inhibits the activation of the TLR4/NF-κB/MAPK pathway and the expression/production of pro-inflammatory cytokines. In summary, SGFD could improve the symptoms and inflammatory response in collagen-induced arthritis (CIA) rat model. The mechanism might be related to the regulation of TLR4/MAPKs/NF-κB signaling pathway and the reduction of inflammatory factor release, which partially confirms the results predicted by network pharmacology.
Keywords: TLR4/MAPKs/NF-κB signaling pathway; inflammatory; network pharmacology; rheumatoid arthritis; shaogan fuzi Decoction.
Copyright © 2022 Shi, Zhao, Feng, Miao, Dong, Wang, Stalin, Zhang, Tu, Liu, Sun and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

