Apatinib for recurrent/progressive glioblastoma multiforme: A salvage option
- PMID: 36060005
- PMCID: PMC9432461
- DOI: 10.3389/fphar.2022.969565
Apatinib for recurrent/progressive glioblastoma multiforme: A salvage option
Abstract
Purpose: The recurrent/progressive glioblastoma multiforme (GBM) carries a dismal prognosis and the definitive treatment strategy has not yet been established. This study aimed to assess the efficacy and safety of apatinib in recurrent/progressive GBM patients. Materials and methods: The clinical data of 19 recurrent/progressive GBM patients who received apatinib treatment from November 2015 to December 2019 at Sun Yat-sen University Cancer Center were collected retrospectively in this study. Objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (AEs) were reviewed and assessed. Results: The overall ORR was 52.6%, and the DCR was 73.7%. Median PFS and OS were 5.1 and 10.4 months, respectively. The 6-month PFS and OS rates were 38.9% and 68.4%, respectively. The 12-month PFS and OS rates were 16.7% and 36.8%, respectively. The treatment-related toxicities were generally well-tolerated. The most common grade 3/4 AEs were hand-foot syndrome (36.8%) and hypertension (21.1%). Conclusion: Our study showed that apatinib therapy provided a better salvaging option for recurrent/progressive GBM patients and the toxicity was manageable.
Keywords: VEGFR; apatinib; efficacy; recurrent/progressive glioblastoma multiforme; safety.
Copyright © 2022 Zhang, Du, Deng, Zheng, Yao, Wang, Yang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
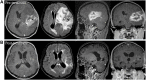
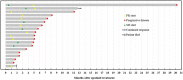
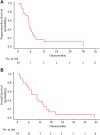
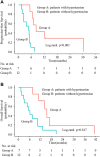
References
-
- Azad N. S., Aragon-Ching J. B., Dahut W. L., Gutierrez M., Figg W. D., Jain L., et al. (2009). Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin. Cancer Res. 15 (4), 1411–1416. 10.1158/1078-0432.CCR-08-1141 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

