U(VI) binding onto electrospun polymers functionalized with phosphonate surfactants
- PMID: 36060014
- PMCID: PMC9435318
- DOI: 10.1016/j.jece.2022.108448
U(VI) binding onto electrospun polymers functionalized with phosphonate surfactants
Abstract
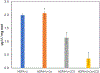
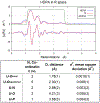
We previously observed that phosphonate functionalized electrospun nanofibers can uptake U(VI), making them promising materials for sensing and water treatment applications. Here, we investigate the optimal fabrication of these materials and their mechanism of U(VI) binding under the influence of environmentally relevant ions (e.g., Ca2+ and ). We found that U(VI) uptake was greatest on polyacrylonitrile (PAN) functionalized with longer-chain phosphonate surfactants (e.g., hexa- and octadecyl phosphonate; HDPA and ODPA, respectively), which were better retained in the nanofiber after surface segregation. Subsequent uptake experiments to better understand specific solid-liquid interfacial interactions were carried out using 5 mg of HDPA-functionalized PAN mats with 10 μM U at pH 6.8 in four systems with different combinations of solutions containing 5 mM calcium (Ca2+) and 5 mM bicarbonate ( ). U uptake was similar in control solutions containing no Ca2+ and (resulting in 19 ± 3% U uptake), and in those containing only 5 mM Ca2+ (resulting in 20 ± 3% U uptake). A decrease in U uptake (10 ± 4% U uptake) was observed in experiments with , indicating that UO2-CO3 complexes may increase uranium solubility. Results from shell-by-shell EXAFS fitting, aqueous extractions, and surface-enhanced Raman scattering (SERS) indicate that U is bound to phosphonate as a monodentate inner sphere surface complex to one of the hydroxyls in the phosphonate functional groups. New knowledge derived from this study on material fabrication and solid-liquid interfacial interactions will help to advance technologies for use in the in-situ detection and treatment of U in water.
Keywords: Electrospun polymer; Phosphonate; Sensing; Spectroscopy; Uranium.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Zychowski K, Pacheco B, Erdei E, Ramone S, Nez T, Gonzales M, Campen MJ, Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities, J. Expo. Sci. Environ. Epidemiol 27 (2017) 365–371. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
