The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): an orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations
- PMID: 36060104
- PMCID: PMC9425786
- DOI: 10.1007/s00044-022-02951-6
The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): an orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations
Abstract
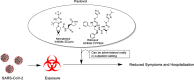
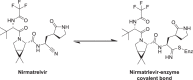
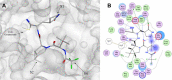
The rapid development of effective vaccines to combat the SARS-CoV-2 virus has been an effective counter measure to decrease hospitalization and the mortality rate in many countries. However, with the risk of mutated strains decreasing the efficacy of the vaccine, there has been an increasing demand for antivirals to treat COVID-19. While antivirals, such as remdesivir, have had some success treating COVID-19 patients in hospital settings, there is a need for orally bioavailable, cost-effective antivirals that can be administered in outpatient settings to minimize COVID-19-related hospitalizations and death. Nirmatrelvir (PF-07321332) is an orally bioavailable Mpro (also called 3CLpro) inhibitor developed by Pfizer. It is administered in combination with ritonavir, a potent CYP3A4 inhibitor that decreases the metabolism of nirmatrelvir. This review seeks to outline the history of the rational design, the target selectivity, synthesis, drug resistance, and future perspectives of nirmatrelvir. Graphical abstract.
Keywords: Antiviral; Main protease; Nirmatrelvir; Paxlovid; SARS-CoV-2.
© The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature 2022, Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures

References
-
- Johns Hopkins Coronavirus Resource Center. 2022. https://coronavirus-jhu-edu.proxy.libraries.rutgers.edu/map.html.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
