Polyvinylpyrrolidone K-30-Based Crosslinked Fast Swelling Nanogels: An Impeccable Approach for Drug's Solubility Improvement
- PMID: 36060130
- PMCID: PMC9439932
- DOI: 10.1155/2022/5883239
Polyvinylpyrrolidone K-30-Based Crosslinked Fast Swelling Nanogels: An Impeccable Approach for Drug's Solubility Improvement
Retraction in
-
Retracted: Polyvinylpyrrolidone K-30-Based Crosslinked Fast Swelling Nanogels: An Impeccable Approach for Drug's Solubility Improvement.Biomed Res Int. 2024 Jan 9;2024:9817465. doi: 10.1155/2024/9817465. eCollection 2024. Biomed Res Int. 2024. PMID: 38230182 Free PMC article.
Abstract
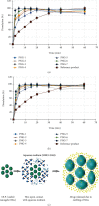
Poor solubility is a global issue of copious pharmaceutical industries as large number of drugs in development stage as well as already marketed products are poorly soluble which results in low dissolution and ultimately dosage increase. Current study is aimed at developing a polyvinylpyrrolidone- (PVP-K30-) based nanogel delivery system for solubility enhancement of poorly soluble drug olanzapine (OLP), as solubilization enhancement is the most noteworthy application of nanosystems. Crosslinking polymerization with subsequent condensation technique was used for the synthesis of nanogels, a highly responsive polymeric networks in drug's solubility. Developed nanogels were characterized by percent entrapment efficiency, sol-gel, percent swelling, percent drug loaded content (%DLC), percent porosity, stability, solubility, in vitro dissolution studies, FTIR, XRD, and SEM analysis. Furthermore, cytotoxicity study was conducted on rabbits to check the biocompatibility of the system. Particle size of nanogels was found with 178.99 ± 15.32 nm, and in vitro dissolution study exhibited that drug release properties were considerably enhanced as compared to the marketed formulation OLANZIA. The solubility studies indicated that solubility of OLP was noticeably improved up to 36.7-fold in phosphate buffer of pH 6.8. In vivo cytotoxicity study indicated that prepared PVP-K30-based formulation was biocompatible. On the basis of results obtained, the developed PVP-K30-co-poly (AMPS) nanogel delivery system is expected to be safe, effective, and cost-effective for solubility improvement of poorly soluble drugs.
Copyright © 2022 Muhammad Usman Minhas et al.
Conflict of interest statement
The authors report no competing interests.
Figures








References
-
- Ambrus R., Szabó-Révész P., Kiss T., et al. Application of a suitable particle engineering technique by pulsed laser ablation in liquid (PLAL) to modify the physicochemical properties of poorly soluble drugs. Journal of Drug Delivery Science and Technology . 2020;57, article 101727 doi: 10.1016/j.jddst.2020.101727. - DOI
-
- Bolourchian N., Nili M., Foroutan S. M., Mahboubi A., Nokhodchi A. The use of cooling and anti-solvent precipitation technique to tailor dissolution and physicochemical properties of meloxicam for better performance. Journal of Drug Delivery Science and Technology . 2020;55, article 101485 doi: 10.1016/j.jddst.2019.101485. - DOI
-
- Sharma M., Sharma R., Jain D. K., Saraf A. Enhancement of oral bioavailability of poorly water soluble carvedilol by chitosan nanoparticles: optimization and pharmacokinetic study. International Journal of Biological Macromolecules . 2019;135:246–260. doi: 10.1016/j.ijbiomac.2019.05.162. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

