Immunotherapy in triple-negative breast cancer: Insights into tumor immune landscape and therapeutic opportunities
- PMID: 36060249
- PMCID: PMC9437219
- DOI: 10.3389/fmolb.2022.903065
Immunotherapy in triple-negative breast cancer: Insights into tumor immune landscape and therapeutic opportunities
Abstract
Triple-negative breast cancer (TNBC) is a clinically aggressive subtype of breast cancer that represents 15-20% of breast tumors and is more prevalent in young pre-menopausal women. It is the subtype of breast cancers with the highest metastatic potential and recurrence at the first 5 years after diagnosis. In addition, mortality increases when a complete pathological response is not achieved. As TNBC cells lack estrogen, progesterone, and HER2 receptors, patients do not respond well to hormone and anti-HER2 therapies, and conventional chemotherapy remains the standard treatment. Despite efforts to develop targeted therapies, this disease continues to have a high unmet medical need, and there is an urgent demand for customized diagnosis and therapeutics. As immunotherapy is changing the paradigm of anticancer treatment, it arises as an alternative treatment for TNBC patients. TNBC is classified as an immunogenic subtype of breast cancer due to its high levels of tumor mutational burden and presence of immune cell infiltrates. This review addresses the implications of these characteristics for the diagnosis, treatment, and prognosis of the disease. Herein, the role of immune gene signatures and tumor-infiltrating lymphocytes as biomarkers in TNBC is reviewed, identifying their application in patient diagnosis and stratification, as well as predictors of efficacy. The expression of PD-L1 expression is already considered to be predictive of response to checkpoint inhibitor therapy, but the challenges regarding its value as biomarker are described. Moreover, the rationales for different formats of immunotherapy against TNBC currently under clinical research are discussed, and major clinical trials are highlighted. Immune checkpoint inhibitors have demonstrated clinical benefit, particularly in early-stage tumors and when administered in combination with chemotherapy, with several regimens approved by the regulatory authorities. The success of antibody-drug conjugates and research on other emerging approaches, such as vaccines and cell therapies, will also be addressed. These advances give hope on the development of personalized, more effective, and safe treatments, which will improve the survival and quality of life of patients with TNBC.
Keywords: biomarkers; immune checkpoint inhibitors; immune gene signatures; immunotherapy; infiltrating T lymphocytes; triple-negative breast cancer; tumor mutational burden.
Copyright © 2022 Ribeiro, Carvalho, Goncalves and Moreira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

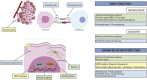
References
-
- Adams S., Gray R. J., Demaria S., Goldstein L., Perez E. A., Shulman L. N., et al. (2014). Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 32 (27), 2959–2966. 10.1200/JCO.2013.55.0491 - DOI - PMC - PubMed
-
- Adams S., Loi S., Toppmeyer D., Cescon D. W., De LaurentiisM., Nanda R., et al. (2019). Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 30 (3), 405–411. 10.1093/annonc/mdy518 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

