CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges
- PMID: 36060553
- PMCID: PMC9439649
- DOI: 10.2147/BLCTT.S327016
CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges
Abstract
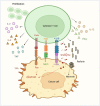
The therapeutic landscape of multiple myeloma (MM) has benefited from an emergence of novel therapies over the last decade. By inducing T-cell kill of target cancer cells, chimeric antigen receptor (CAR) T-cell therapies have improved outcomes of patients with hematologic malignancies. B-cell maturation antigen (BCMA) is the current target antigen of choice for most CAR T-cell products under investigation for MM. However, their shortcomings deal with logistical and clinical challenges, including limited availability, manufacturing times, and toxicities. This article provides an overview of recently developed and investigational CAR T-cell therapies for MM, highlighting current evidence and challenges.
Keywords: CAR T-cell; immunotherapy; multiple myeloma.
© 2022 Rendo et al.
Conflict of interest statement
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the US Air Force or the Department of Defense. None of the authors have any financial or non-financial conflicts of interest to disclose.
Figures
References
-
- National Cancer Institute. Cancer stat facts: myeloma 2021. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed August 22, 2022.
-
- Sharpe AH, Abbas AK. T-cell costimulation–biology, therapeutic potential, and challenges. N Engl J Med. 2006;355(10):973–975. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials