Evaluation of two different vaccine platforms for immunization against melioidosis and glanders
- PMID: 36060742
- PMCID: PMC9428723
- DOI: 10.3389/fmicb.2022.965518
Evaluation of two different vaccine platforms for immunization against melioidosis and glanders
Abstract
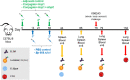
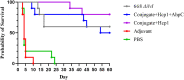
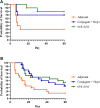
Burkholderia pseudomallei and the closely related species, Burkholderia mallei, produce similar multifaceted diseases which range from rapidly fatal to protracted and chronic, and are a major cause of mortality in endemic regions. Besides causing natural infections, both microbes are Tier 1 potential biothreat agents. Antibiotic treatment is prolonged with variable results, hence effective vaccines are urgently needed. The purpose of our studies was to compare candidate vaccines that target both melioidosis and glanders to identify the most efficacious one(s) and define residual requirements for their transition to the non-human primate aerosol model. Studies were conducted in the C57BL/6 mouse model to evaluate the humoral and cell-mediated immune response and protective efficacy of three Burkholderia vaccine candidates against lethal aerosol challenges with B. pseudomallei K96243, B. pseudomallei MSHR5855, and B. mallei FMH. The recombinant vaccines generated significant immune responses to the vaccine antigens, and the live attenuated vaccine generated a greater immune response to OPS and the whole bacterial cells. Regardless of the candidate vaccine evaluated, the protection of mice was associated with a dampened cytokine response within the lungs after exposure to aerosolized bacteria. Despite being delivered by two different platforms and generating distinct immune responses, two experimental vaccines, a capsule conjugate + Hcp1 subunit vaccine and the live B. pseudomallei 668 ΔilvI strain, provided significant protection and were down-selected for further investigation and advanced development.
Keywords: Burkholderia mallei; Burkholderia pseudomallei; aerosol; cellular immunity; humoral immunity; immune response; mice; vaccine.
Copyright © 2022 Biryukov, Cote, Klimko, Dankmeyer, Rill, Shoe, Hunter, Shamsuddin, Velez, Hedrick, Rosario-Acevedo, Talyansky, Schmidt, Orne, Fetterer, Burtnick, Brett, Welkos and DeShazer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Amemiya K., Dankmeyer J. L., Biryukov S. S., Trevino S. R., Klimko C. P., Mou S. M., et al. . (2019). Deletion of two genes in Burkholderia pseudomallei MSHR668 that target essential amino acids protect acutely infected BALB/c mice and promote long term survival. Vaccines 7, 196. 10.3390/vaccines7040196 - DOI - PMC - PubMed
-
- Atkins T., Prior R. G., Mack K., Russell P., Nelson M., Oyston P. C., et al. . (2002). A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect. Immun. 70, 5290–5294. 10.1128/IAI.70.9.5290-5294.2002 - DOI - PMC - PubMed
-
- AuCoin D. P., Reed D. E., Marlenee N. L., Bowen R. A., Thorkildson P., Judy B. M., et al. . (2012). Polysaccharide specific monoclonal antibodies provide passive protection against intranasal challenge with Burkholderia pseudomallei. PLoS ONE 7, e35386. 10.1371/journal.pone.0035386 - DOI - PMC - PubMed
-
- Baker S. M., Davitt C. J. H., Motyka N., Kikendall N. L., Russell-Lodrigue K., Roy C. J., et al. . (2017). A Burkholderia pseudomallei outer membrane vesicle vaccine provides cross protection against inhalational glanders in mice and non-human primates. Vaccines 5, 49. 10.3390/vaccines5040049 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

