Evaluation of the biotechnological potential of peptide Cupiennin 1a and analogs
- PMID: 36060778
- PMCID: PMC9433906
- DOI: 10.3389/fmicb.2022.850007
Evaluation of the biotechnological potential of peptide Cupiennin 1a and analogs
Abstract
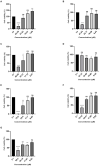
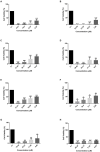
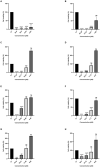
Antimicrobial peptides (AMPs) are components in the innate immune system of various organisms, and many AMPs can be found in poisons from animals such as spiders, scorpions, and snakes. The peptide Cupiennin-1a is present in the venom of the spider Cupiennius salei and belongs to a group of peptides called cupiennins. The peptide demonstrated high cytotoxic activity against mammalian cells; thus, aiming to solve this problem, seven analogs were designed (R1a, R1b, R2b, R3b, R6b, R8b, and R10b) based on the primary structure of the peptide Cupiennin 1a, reducing its size and substituting some amino acid residues. The antimicrobial results showed that all Cupiennin 1a analogs displayed antimicrobial activity against the tested bacterial and fungal strains. Cytotoxicity tests demonstrated a decrease in the cytotoxic effect of the analogs when compared to the peptide Cupiennin-1a. The antitumor activity against breast adenocarcinoma lines was observed for all the peptides, displaying a better effect against the MCF-7 and MDAMB-231 cell lines. The eight peptides have insecticidal potential, and the original peptide and analogs R6b, R8b, and R10b showed better efficiency even at low concentrations. The rational design of the analogs led to new molecules displaying activities against different cell types and reduced cytotoxicity toward healthy mammalian cells when compared to the original peptide, demonstrating that this was an interesting approach for the development of molecules with biotechnological potential.
Keywords: analogs; antimicrobial peptides; cytotoxicity; rational design; spider.
Copyright © 2022 Araújo, Leite, Dutra, Brito da Cunha, Rezende, Ramada and Dias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Cupiennin 1, a new family of highly basic antimicrobial peptides in the venom of the spider Cupiennius salei (Ctenidae).J Biol Chem. 2002 Mar 29;277(13):11208-16. doi: 10.1074/jbc.M111099200. Epub 2002 Jan 15. J Biol Chem. 2002. PMID: 11792701
-
N-terminal aromatic residues closely impact the cytolytic activity of cupiennin 1a, a major spider venom peptide.Toxicon. 2013 Dec 1;75:177-86. doi: 10.1016/j.toxicon.2013.03.003. Epub 2013 Mar 21. Toxicon. 2013. PMID: 23523532
-
Cupiennin 1a, an antimicrobial peptide from the venom of the neotropical wandering spider Cupiennius salei, also inhibits the formation of nitric oxide by neuronal nitric oxide synthase.FEBS J. 2007 Apr;274(7):1778-84. doi: 10.1111/j.1742-4658.2007.05726.x. Epub 2007 Feb 22. FEBS J. 2007. PMID: 17313650
-
Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae).Toxicon. 2004 Apr;43(5):543-53. doi: 10.1016/j.toxicon.2004.02.009. Toxicon. 2004. PMID: 15066412 Review.
-
Antimicrobial Peptides From Lycosidae (Sundevall, 1833) Spiders.Curr Protein Pept Sci. 2020;21(5):527-541. doi: 10.2174/1389203721666200116091911. Curr Protein Pept Sci. 2020. PMID: 31951167 Review.
References
-
- Adem Bahar A. (2015). Controlling Biofilm and Persister Cells By Targeting Cell Membranes. Control Biofilm Persister Cells By Target Cell Membrane. Available at: https://surface.syr.edu/etd/377
-
- Almsned F. (2017). Designing antimicrobial peptide: current status. J. Med. Sci. Clin. Res. 05, 19282–19294. doi: 10.18535/jmscr/v5i3.153 - DOI
-
- Castro M. E. B., Ribeiro Z. M. A., Souza M. L. (2006). Infectivity of Anticarsia gemmatalis nucleopolyhedrovirus to different insect cell lines: morphology, viral production, and protein synthesis. Biol. Control 36, 299–304. doi: 10.1016/j.biocontrol.2005.10.002 - DOI
-
- Clinical Laboratory Standards Institute . (2008). Reference method broth dilution Antifungal susceptibility Test of Yeasts. Approved Standard 3rd Edition. 28, 0–13. Available at: https://clsi.org/media/1461/m27a3_sample.pdf
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

