Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
- PMID: 36060972
- PMCID: PMC9434279
- DOI: 10.3389/fendo.2022.976465
Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
Abstract
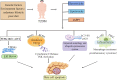
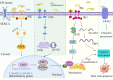
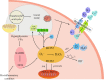
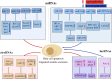
Type 2 diabetes mellitus(T2DM) is regarded as one of the most severe chronic metabolic diseases worldwide, which poses a great threat to human safety and health. The main feature of T2DM is the deterioration of pancreatic beta-cell function. More and more studies have shown that the decline of pancreatic beta-cell function in T2DM can be attributable to beta-cell apoptosis, but the exact mechanisms of beta-cell apoptosis in T2DM are not yet fully clarified. Therefore, in this review, we will focus on the current status and progress of research on the mechanism of pancreatic beta-cell apoptosis in T2DM, to provide new ideas for T2DM treatment strategies.
Keywords: apoptosis; beta-cell; exosomes; glucolipotoxicity; molecular mechanisms; non-coding RNAs; type 2 diabetes.
Copyright © 2022 You, Zheng, Chen and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction.ACS Nano. 2018 Aug 28;12(8):7613-7628. doi: 10.1021/acsnano.7b07643. Epub 2018 Aug 7. ACS Nano. 2018. PMID: 30052036
-
Pancreatic fat content is associated with β-cell function and insulin resistance in Chinese type 2 diabetes subjects.Endocr J. 2019 Mar 28;66(3):265-270. doi: 10.1507/endocrj.EJ18-0436. Epub 2019 Jan 30. Endocr J. 2019. PMID: 30700664
-
Relationships between type 2 diabetes, cell dysfunction, and redox signaling: A meta-analysis of single-cell gene expression of human pancreatic α- and β-cells.J Diabetes. 2022 Jan;14(1):34-51. doi: 10.1111/1753-0407.13236. Epub 2021 Dec 13. J Diabetes. 2022. PMID: 34725923 Free PMC article.
-
Insights on the current status and advancement of diabetes mellitus type 2 and to avert complications: An overview.Biotechnol Appl Biochem. 2020 Nov;67(6):920-928. doi: 10.1002/bab.1853. Epub 2020 Feb 25. Biotechnol Appl Biochem. 2020. PMID: 31736194 Review.
-
What role do fat cells play in pancreatic tissue?Mol Metab. 2019 Jul;25:1-10. doi: 10.1016/j.molmet.2019.05.001. Epub 2019 May 7. Mol Metab. 2019. PMID: 31113756 Free PMC article. Review.
Cited by
-
Single and Combined Impact of Semaglutide, Tirzepatide, and Metformin on β-Cell Maintenance and Function Under High-Glucose-High-Lipid Conditions: A Comparative Study.Int J Mol Sci. 2025 Jan 6;26(1):421. doi: 10.3390/ijms26010421. Int J Mol Sci. 2025. PMID: 39796271 Free PMC article.
-
Excess Fructose Intake Activates Hyperinsulinemia and Mitogenic MAPK Pathways in Association With Cellular Stress, Inflammation, and Apoptosis in the Pancreas of Rats.Mol Nutr Food Res. 2025 May;69(10):e70048. doi: 10.1002/mnfr.70048. Epub 2025 Mar 28. Mol Nutr Food Res. 2025. PMID: 40152093 Free PMC article.
-
Role of cytosolic and endoplasmic reticulum Ca2+ in pancreatic beta-cells: pros and cons.Pflugers Arch. 2024 Feb;476(2):151-161. doi: 10.1007/s00424-023-02872-2. Epub 2023 Nov 9. Pflugers Arch. 2024. PMID: 37940681 Review.
-
Targeting Protein Kinases to Protect Beta-Cell Function and Survival in Diabetes.Int J Mol Sci. 2024 Jun 11;25(12):6425. doi: 10.3390/ijms25126425. Int J Mol Sci. 2024. PMID: 38928130 Free PMC article. Review.
-
Effects of different hypoglycaemic drugs on beta-cell function in type 2 diabetes mellitus: a systematic review and network meta-analysis.Eur J Med Res. 2025 Feb 21;30(1):121. doi: 10.1186/s40001-025-02368-y. Eur J Med Res. 2025. PMID: 39985051 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

