Vibration therapy as an effective approach to improve bone healing in diabetic rats
- PMID: 36060973
- PMCID: PMC9437439
- DOI: 10.3389/fendo.2022.909317
Vibration therapy as an effective approach to improve bone healing in diabetic rats
Abstract
Objective: To investigate the effects of vibration therapy on fracture healing in diabetic and non-diabetic rats.
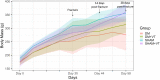
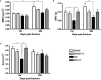
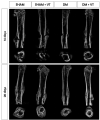
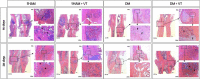
Methods: 148 rats underwent fracture surgery and were assigned to four groups: (1) SHAM: weight-matched non-diabetic rats, (2) SHAM+VT: non-diabetic rats treated with vibration therapy (VT), (3) DM: diabetic rats, and (4) DM+VT: diabetic rats treated with VT. Thirty days after diabetes induction with streptozotocin, animals underwent bone fracture, followed by surgical stabilization. Three days after bone fracture, rats began VT. Bone healing was assessed on days 14 and 28 post-fracture by serum bone marker analysis, and femurs collected for dual-energy X-ray absorptiometry, micro-computed tomography, histology, and gene expression.
Results: Our results are based on 88 animals. Diabetes led to a dramatic impairment of bone healing as demonstrated by a 17% reduction in bone mineral density and decreases in formation-related microstructural parameters compared to non-diabetic control rats (81% reduction in bone callus volume, 69% reduction in woven bone fraction, 39% reduction in trabecular thickness, and 45% in trabecular number). These changes were accompanied by a significant decrease in the expression of osteoblast-related genes (Runx2, Col1a1, Osx), as well as a 92% reduction in serum insulin-like growth factor I (IGF-1) levels. On the other hand, resorption-related parameters were increased in diabetic rats, including a 20% increase in the callus porosity, a 33% increase in trabecular separation, and a 318% increase in serum C terminal telopeptide of type 1 collagen levels. VT augmented osteogenic and chondrogenic cell proliferation at the fracture callus in diabetic rats; increased circulating IGF-1 by 668%, callus volume by 52%, callus bone mineral content by 90%, and callus area by 72%; and was associated with a 19% reduction in circulating receptor activator of nuclear factor kappa beta ligand (RANK-L).
Conclusions: Diabetes had detrimental effects on bone healing. Vibration therapy was effective at counteracting the significant disruption in bone repair induced by diabetes, but did not improve fracture healing in non-diabetic control rats. The mechanical stimulus not only improved bone callus quality and quantity, but also partially restored the serum levels of IGF-1 and RANK-L, inducing bone formation and mineralization, thus creating conditions for adequate fracture repair in diabetic rats.
Keywords: bone turnover; bony callus; diabetes mellitus; fracture healing; vibration.
Copyright © 2022 Campos, Volpon, Ximenez, Franttini, Dalloul, Sousa-Neto, Silva, Kacena and Zamarioli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

