LC-MS/MS standardization and validation of glycyrrhizin from the roots of Taverniera cuneifolia: A potential alternative source of Glycyrrhiza glabra
- PMID: 36061022
- PMCID: PMC9429499
- DOI: 10.1016/j.heliyon.2022.e10234
LC-MS/MS standardization and validation of glycyrrhizin from the roots of Taverniera cuneifolia: A potential alternative source of Glycyrrhiza glabra
Abstract
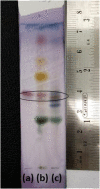
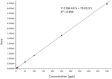
Glycyrrhizin is a triterpene glycoside derived from Glycyrrhiza glabra and related species which is a renowned phytochemical used to cure a variety of ailments such as inflammation, sore throat, hepatitis etc. It is in huge demand owing to its various valuable properties. With the ever-increasing demand of glycyrrhizin, the search for alternative sources for glycyrrhizin is on rise. One such species with a scientific basis and good concentration of glycyrrhizin is Taverniera cuneifolia. A thin-layer chromatography (TLC) method was established to determine the presence of glycyrrhizin in T. cuneifolia. Further, standardisation and validation, a High performance liquid chromatography (model NEXERA-X2) with LCMS system (Model LCMS-8040) from Shimadzu were used. The analysis was performed by using shim-pack XR-ODS, C18 (75 mm × 3.0 mm) 2.2 μm. In this analysis, the mobile phase used was a combination of acetonitrile and a 20 mM ammonium acetate buffer that was subjected to gradient time programming and monitored by Multiple Reaction Monitoring (MRM) in positive ion mode. The method was validated for linearity, accuracy, precision, recovery, detection, and quantitation limit. The technique was confirmed to be linear within the concentration range of 5 ng/mL to 500 ng/mL with R2 > 0.991. The LOD and the LOQ were 2 ng/mL and 5 ng/mL respectively. The suggested approach satisfied the acceptance criteria for linearity, accuracy, precision, specificity, robustness, LOD, LOQ, and system adaptability.
Keywords: Glycyrrhizin; LC-MS/MS; Licorice; Phytoconstituents; Taverniera cuneifolia.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Abe N., Ebina T., Ishida N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol. Immunol. 1982;26(6):535–539. - PubMed
-
- Yasui S., Fujiwara K., Tawada A., Fukuda Y., Nakano M., Yokosuka O. Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis. Dig. Dis. Sci. 2011;56(12):3638–3647. - PubMed
-
- Arase Y., Ikeda K., Murashima N., Chayama K., Tsubota A., Koida I., et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79(8):1494–1500. - PubMed
-
- Ikeda K. Glycyrrhizin injection therapy prevents hepatocellular carcinogenesis in patients with interferon-resistant active chronic hepatitis C. Hepatol. Res.: Off. J. Jpn. Soc. Hepatol. 2007;37(Suppl 2):S287–S293. - PubMed
LinkOut - more resources
Full Text Sources

