Prevention of malaria in pregnancy: The threat of sulfadoxine-pyrimethamine resistance
- PMID: 36061376
- PMCID: PMC9433640
- DOI: 10.3389/fped.2022.966402
Prevention of malaria in pregnancy: The threat of sulfadoxine-pyrimethamine resistance
Abstract
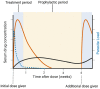
Malaria infection in pregnancy can lead to adverse outcomes for both the pregnant person and fetus. The administration of intermittent preventative therapy (IPTp) with sulfadoxine-pyrimethamine (SP) during pregnancy (IPTp-SP) improves outcomes, including severe maternal anemia, placental malaria infection, and low infant birth weight. The WHO recommends IPTp-SP for pregnant individuals living in areas of moderate or high malaria transmission in Africa. The current regimen consists of two or more doses of SP starting as early as possible in the second trimester, at least 1 month apart. Unfortunately, rising Plasmodium falciparum SP resistance throughout Africa threatens to erode the benefits of SP. Recent studies have shown a decrease in IPTp-SP efficacy in areas with high SP resistance. Thus, there is an urgent need to identify new drug regimens that can be used for intermittent preventative therapy in pregnancy. In this review, we discuss recent data on P. falciparum SP resistance in Africa, the effect of resistance on IPTp-SP, and studies of alternative IPTp regimens. Finally, we present a framework for the ideal pharmacokinetic and pharmacodynamic properties for future IPTp regimens.
Keywords: IPTp; antimalarial; drug resistance; low birth weight; malaria.
Copyright © 2022 Sundararaman and Odom John.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization (WHO) . World Malaria Report 2021. Geneva: World Health Organization; (2021).
LinkOut - more resources
Full Text Sources

