Multiple propane gas burn rates procedure to determine accuracy and linearity of indirect calorimetry systems: an experimental assessment of a method
- PMID: 36061755
- PMCID: PMC9435516
- DOI: 10.7717/peerj.13882
Multiple propane gas burn rates procedure to determine accuracy and linearity of indirect calorimetry systems: an experimental assessment of a method
Abstract
Objective: Indirect calorimetry (IC) systems measure the fractions of expired carbon dioxide (FeCO2), and oxygen (FeO2) recorded at the mouth to estimate whole-body energy production. The fundamental principle of IC relates to the catabolism of high-energy substrates such as carbohydrates and lipids to meet the body's energy needs through the oxidative process, which are reflected in the measured oxygen uptake rates (V̇O2) and carbon dioxide production rates (V̇CO2). Accordingly, it is important to know the accuracy and validity of V̇O2and V̇CO2 measurements when estimating energy production and substrate partitioning for research and clinical purposes. Although several techniques are readily available to assess the accuracy of IC systems at a single point for V̇CO2 and V̇O2, the validity of such procedures is limited when used in testing protocols that incorporate a wide range of energy production (e.g., basal metabolic rate and maximal exercise testing). Accordingly, we built an apparatus that allowed us to manipulate propane burn rates in such a way as to assess the linearity of IC systems. This technical report aimed to assess the accuracy and linearity of three IC systems using our in-house built validation procedure.
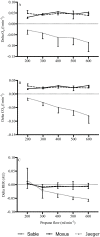
Approach: A series of trials at different propane burn rates (PBR) (i.e., 200, 300, 400, 500, and 600 mL min-1) were run on three IC systems: Sable, Moxus, and Oxycon Pro. The experimental values for V̇O2 and V̇CO2 measured on the three IC systems were compared to theoretical stoichiometry values.
Results: A linear relationship was observed between increasing PBR and measured values for V̇O2and V̇CO2 (99.6%, 99.2%, 94.8% for the Sable, Moxus, and Jaeger IC systems, respectively). In terms of system error, the Jaeger system had significantly (p < 0.001) greater V̇O2(mean difference (M) = -0.057, standard error (SE) = 0.004), and V̇CO2(M = -0.048, SE = 0.002) error compared to either the Sable (V̇O2, M = 0.044, SE = 0.004; V̇CO2, M = 0.024, SE = 0.002) or the Moxus (V̇O2, M = 0.046, SE = 0.004; V̇CO2, M = 0.025, SE = 0.002) IC systems. There were no significant differences between the Sable or Moxus IC systems.
Conclusion: The multiple PBR approach permitted the assessment of linearity of IC systems in addition to determining the accuracy of fractions of expired gases.
Keywords: Accuracy; Energy production; Indirect calorimetry; Linearity; Propane gas.
©2022 Ismail et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Brooks GA, Fahey TD, Baldwin KM. Exercise physiology: human bioenergetics and its applications. 4th ed McGraw-Hill; Boston: 2005.
-
- Cooper CB, Storer TW. Exercise testing and interpretation: a practical approach. Cambridge University Press; Cambridge, UK; New York, NY, USA: 2001.
-
- Field AP. Discovering statistics using IBM SPSS statistics. SAGE Publications, Limited; Toronto: 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

