A microarray patch SARS-CoV-2 vaccine induces sustained antibody responses and polyfunctional cellular immunity
- PMID: 36062075
- PMCID: PMC9425707
- DOI: 10.1016/j.isci.2022.105045
A microarray patch SARS-CoV-2 vaccine induces sustained antibody responses and polyfunctional cellular immunity
Abstract
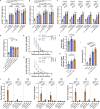
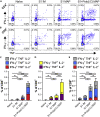
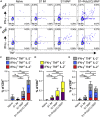
Sustainable global immunization campaigns against COVID-19 and other emerging infectious diseases require effective, broadly deployable vaccines. Here, we report a dissolvable microarray patch (MAP) SARS-CoV-2 vaccine that targets the immunoresponsive skin microenvironment, enabling efficacious needle-free immunization. Multicomponent MAPs delivering both SARS-CoV-2 S1 subunit antigen and the TLR3 agonist Poly(I:C) induce robust antibody and cellular immune responses systemically and in the respiratory mucosa. MAP vaccine-induced antibodies bind S1 and the SARS-CoV-2 receptor-binding domain, efficiently neutralize the virus, and persist at high levels for more than a year. The MAP platform reduces systemic toxicity of the delivered adjuvant and maintains vaccine stability without refrigeration. When applied to human skin, MAP vaccines activate skin-derived migratory antigen-presenting cells, supporting the feasibility of human translation. Ultimately, this shelf-stable MAP vaccine improves immunogenicity and safety compared to traditional intramuscular vaccines and offers an attractive alternative for global immunization efforts against a range of infectious pathogens.
Keywords: Immunology; Medical biotechnology; Virology.
© 2022 The Author(s).
Conflict of interest statement
E. Korkmaz and L.D. Falo Jr. are inventors of related intellectual property. L.D. Falo Jr. is a co-founder and scientific advisor of SkinJect, a company that is developing dissolvable MAPs for treatment of non-melanoma skin cancer.
Figures



Similar articles
-
Long-term Immunity of a Microneedle Array Patch of SARS-CoV-2 S1 Protein Subunit Vaccine Irradiated by Gamma Rays in Mice.bioRxiv [Preprint]. 2024 Oct 25:2024.10.25.620289. doi: 10.1101/2024.10.25.620289. bioRxiv. 2024. Update in: Vaccines (Basel). 2025 Jan 18;13(1):86. doi: 10.3390/vaccines13010086. PMID: 39484497 Free PMC article. Updated. Preprint.
-
The Long-Term Immunity of a Microneedle Array Patch of a SARS-CoV-2 S1 Protein Subunit Vaccine Irradiated by Gamma Rays in Mice.Vaccines (Basel). 2025 Jan 18;13(1):86. doi: 10.3390/vaccines13010086. Vaccines (Basel). 2025. PMID: 39852865 Free PMC article.
-
Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development.EBioMedicine. 2020 May;55:102743. doi: 10.1016/j.ebiom.2020.102743. Epub 2020 Apr 2. EBioMedicine. 2020. PMID: 32249203 Free PMC article.
-
Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase I clinical trial.PLoS Med. 2020 Mar 17;17(3):e1003024. doi: 10.1371/journal.pmed.1003024. eCollection 2020 Mar. PLoS Med. 2020. PMID: 32181756 Free PMC article. Clinical Trial.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
Long-term Immunity of a Microneedle Array Patch of SARS-CoV-2 S1 Protein Subunit Vaccine Irradiated by Gamma Rays in Mice.bioRxiv [Preprint]. 2024 Oct 25:2024.10.25.620289. doi: 10.1101/2024.10.25.620289. bioRxiv. 2024. Update in: Vaccines (Basel). 2025 Jan 18;13(1):86. doi: 10.3390/vaccines13010086. PMID: 39484497 Free PMC article. Updated. Preprint.
-
Fourth dose of microneedle array patch of SARS-CoV-2 S1 protein subunit vaccine elicits robust long-lasting humoral responses in mice.Int Immunopharmacol. 2024 Mar 10;129:111569. doi: 10.1016/j.intimp.2024.111569. Epub 2024 Feb 9. Int Immunopharmacol. 2024. PMID: 38340419 Free PMC article.
-
The Long-Term Immunity of a Microneedle Array Patch of a SARS-CoV-2 S1 Protein Subunit Vaccine Irradiated by Gamma Rays in Mice.Vaccines (Basel). 2025 Jan 18;13(1):86. doi: 10.3390/vaccines13010086. Vaccines (Basel). 2025. PMID: 39852865 Free PMC article.
References
-
- Bandyopadhyay M., Morelli A.E., Balmert S.C., Ward N.L., Erdos G., Sumpter T.L., Korkmaz E., Kaplan D.H., Oberbarnscheidt M.H., Tkacheva O. Skin co-delivery of contact sensitizers and neurokinin-1 receptor antagonists integrated in microneedle arrays suppresses allergic contact dermatitis. J. Allergy Clin. Immunol. 2022;150:114–130. doi: 10.1016/j.jaci.2021.12.794. - DOI - PMC - PubMed
-
- Bardel E., Doucet-Ladeveze R., Mathieu C., Harandi A.M., Dubois B., Kaiserlian D. Intradermal immunisation using the TLR3-ligand Poly (I:C) as adjuvant induces mucosal antibody responses and protects against genital HSV-2 infection. NPJ Vaccines. 2016;1:16010. doi: 10.1038/npjvaccines.2016.10. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

