Microbiota manipulation to increase macrophage IL-10 improves colitis and limits colitis-associated colorectal cancer
- PMID: 36062329
- PMCID: PMC9450902
- DOI: 10.1080/19490976.2022.2119054
Microbiota manipulation to increase macrophage IL-10 improves colitis and limits colitis-associated colorectal cancer
Abstract
Inflammatory bowel disease (IBD) is a chronic life-long inflammatory disease affecting almost 2 million Americans. Although new biologic therapies have been developed, the standard medical treatment fails to selectively control the dysregulated immune pathways involved in chronic colonic inflammation. Further, IBD patients with uncontrolled colonic inflammation are at a higher risk for developing colorectal cancer (CRC). Intestinal microbes can impact many immune functions, and here we asked if they could be used to improve intestinal inflammation. By utilizing an intestinal adherent E. coli that we find increases IL-10 producing macrophages, we were able to limit intestinal inflammation and restrict tumor formation. Macrophage IL-10 along with IL-10 signaling to the intestinal epithelium were required for protection in both inflammation and tumor development. Our work highlights that administration of immune modulating microbes can improve intestinal outcomes by altering tissue inflammation.
Keywords: E. coli; IL-10; Microbiota; colitis-associated cancer; colorectal cancer; intestinal epithelium; intestinal inflammation; intestinal macrophages.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

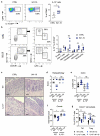


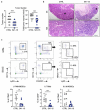
References
-
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, Sadeghi A, Nixon MR, Abdoli A, Abolhassani H, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterology Hepatology. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
