HCV hotline facilitates Hepatitis C elimination during the COVID-19 pandemic
- PMID: 36062398
- PMCID: PMC9825935
- DOI: 10.1111/jvh.13746
HCV hotline facilitates Hepatitis C elimination during the COVID-19 pandemic
Abstract
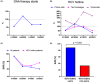
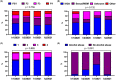
The COVID-19 pandemic necessitates healthcare restrictions that also affected ongoing hepatitis C virus (HCV) elimination efforts. We assessed the value of a physician-operated HCV hotline on treatment and cure rates throughout the pandemic. All HCV patients undergoing HCV therapy at the Vienna General Hospital from 2019 to 2021 were included. An HCV hotline was established in 2019 and provided services including phone calls, text messages and voicemails. Patients were stratified by date of HCV therapy: 2019 (pre-COVID) vs. 2020/2021 (during-COVID) and use of the HCV hotline: users vs. non-users. Overall, 220 patients were included (pre-COVID: n = 91 vs. during-COVID: n = 129). The prevalence of intravenous drug use (60.5%) and alcohol abuse (24.8%) was high during COVID. During COVID, the number of DAA treatment starts declined by 24.2% (n = 69) in 2020 and by 34.1% (n = 60) in 2021 vs. pre-COVID (n = 91, 100%). Significantly more patients used the HCV hotline during-COVID (95.3%) vs. pre-COVID (65.9%; p < .001). Sustained virologic response (SVR) was 84.6% pre-COVID and 86.0% during-COVID. HCV hotline users achieved higher SVR rates during-COVID (88.2% vs. 33.3%, p = .004), but also pre-COVID (96.7% vs. 61.3%, p < .001) compared with non-users. Considering only patients with completed DAA treatments, SVR rates remained similarly high during-COVID (96.9%) versus pre-COVID (98.1%). HCV treatment initiations decreased during-COVID but importantly, nearly all DAA-treated HCV patients used the HCV hotline during the COVID pandemic. Overall, the SVR rate remained at 88.2% during COVID and was particularly high in HCV phone users-most likely due to facilitation of adherence.
Keywords: COVID-19; elimination; hepatitis C virus; telemedicine.
© 2022 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
L.H., M.J., D.C., T.B., L.S., C.S., M.S., L.B. and R.S. have nothing to disclose. D.B. received speaker fees from AbbVie and Siemens, as well as grant support form Gilead and Siemens, as well as travel support from AbbVie and Gilead. B.Sim. received travel support from AbbVie and Gilead. M.T. served as a speaker and/or consultant and/or advisory board member for Albireo, BiomX, Falk, Boehringer Ingelheim, Bristol‐Myers Squibb, Falk, Genfit, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, and Shire, and received travel support from AbbVie, Falk, Gilead, and Intercept, as well as grants/research support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx. He is also co‐inventor of patents on the medical use of 24‐norursodeoxycholic acid. M.G. received grants from AbbVie, Gilead and MSD; speaking honoraria/advisory board fees from AbbVie, Gilead, MSD, Janssen, BMS, Roche, Intercept, Norgine, AstraZeneca, Alnylam, Falk and Shionogi. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Gilead, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. T.R. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Pliant, Philips, Siemens and W. L. Gore & Associates as well as travel support from AbbVie, Boehringer Ingelheim, Gilead and Roche.
Figures

Comment in
-
Monkeypox outbreak as an opportunity to identify new cases of HCV infection in limited resource settings.J Viral Hepat. 2023 Jan;30(1):83-85. doi: 10.1111/jvh.13771. Epub 2022 Nov 17. J Viral Hepat. 2023. PMID: 36357317 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

