Adjuvant atezolizumab in Japanese patients with resected stage IB-IIIA non-small cell lung cancer (IMpower010)
- PMID: 36062851
- PMCID: PMC9746048
- DOI: 10.1111/cas.15564
Adjuvant atezolizumab in Japanese patients with resected stage IB-IIIA non-small cell lung cancer (IMpower010)
Erratum in
-
Correction.Cancer Sci. 2023 May;114(5):2211-2212. doi: 10.1111/cas.15711. Epub 2023 Feb 15. Cancer Sci. 2023. PMID: 36791690 Free PMC article. No abstract available.
Abstract
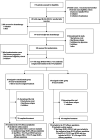
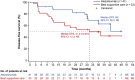
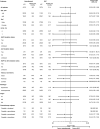
The global phase 3 IMpower010 study evaluated adjuvant atezolizumab versus best supportive care (BSC) following platinum-based chemotherapy in patients with resected stage IB-IIIA non-small cell lung cancer (NSCLC). Here, we report a subgroup analysis in patients enrolled in Japan. Eligible patients had complete resection of histologically or cytologically confirmed stage IB (tumors ≥4 cm)-IIIA NSCLC. Upon completing 1-4 cycles of adjuvant cisplatin-based chemotherapy, patients were randomized 1:1 to receive atezolizumab (fixed dose of 1200 mg every 21 days; 16 cycles or 1 year) or BSC. The primary endpoint of the global IMpower010 study was investigator-assessed disease-free survival, tested hierarchically first in patients with stage II-IIIA NSCLC whose tumors expressed programmed death-ligand 1 (PD-L1) on ≥1% of tumor cells, then in all randomized patients with stage II-IIIA NSCLC, and finally in the intention-to-treat (ITT) population (stage IB-IIIA NSCLC). Safety was evaluated in all patients who received atezolizumab or BSC. The study comprised 149 enrolled patients in three populations: ITT (n = 117; atezolizumab, n = 59; BSC, n = 58), all-randomized stage II-IIIA (n = 113; atezolizumab, n = 56; BSC, n = 57), and PD-L1 tumor cells ≥1% stage II-IIIA (n = 74; atezolizumab, n = 41; BSC, n = 33). At the data cutoff date (January 21, 2021), a trend toward disease-free survival improvement with atezolizumab vs BSC was observed in the PD-L1 tumor cells ≥1% stage II-IIIA (unstratified hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.25-1.08), all-randomized stage II-IIIA (unstratified HR, 0.62; 95% CI, 0.35-1.11), and ITT (unstratified HR, 0.61; 95% CI, 0.34-1.10) populations. Atezolizumab-related grade 3/4 adverse events occurred in 16% of patients; no treatment-related grade 5 events occurred. Adjuvant atezolizumab showed disease-free survival improvement and a tolerable toxicity profile in Japanese patients in IMpower010, consistent with the global study results.
Keywords: Japanese; PD-L1 inhibitor; PD-L1 protein; atezolizumab; non-small cell lung cancer.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
Similar articles
-
Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial.Lancet. 2021 Oct 9;398(10308):1344-1357. doi: 10.1016/S0140-6736(21)02098-5. Epub 2021 Sep 20. Lancet. 2021. PMID: 34555333 Clinical Trial.
-
Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial.Ann Oncol. 2023 Oct;34(10):907-919. doi: 10.1016/j.annonc.2023.07.001. Epub 2023 Jul 17. Ann Oncol. 2023. PMID: 37467930 Clinical Trial.
-
Five-Year Survival Outcomes With Atezolizumab After Chemotherapy in Resected Stage IB-IIIA Non-Small Cell Lung Cancer (IMpower010): An Open-Label, Randomized, Phase III Trial.J Clin Oncol. 2025 Jul 20;43(21):2343-2349. doi: 10.1200/JCO-24-01681. Epub 2025 May 30. J Clin Oncol. 2025. PMID: 40446184 Clinical Trial.
-
Atezolizumab Monotherapy or Plus Chemotherapy in First-Line Treatment for Advanced Non-Small Cell Lung Cancer Patients: A Meta-Analysis.Front Immunol. 2021 Jun 2;12:666909. doi: 10.3389/fimmu.2021.666909. eCollection 2021. Front Immunol. 2021. PMID: 34149702 Free PMC article.
-
Immune checkpoint inhibitors as adjuvant therapy in patients with completely resected nonsmall cell lung cancer.Curr Opin Oncol. 2024 Jan 1;36(1):24-28. doi: 10.1097/CCO.0000000000001003. Epub 2023 Oct 20. Curr Opin Oncol. 2024. PMID: 37865822 Review.
Cited by
-
The Effect of Sex on the Therapeutic Efficiency of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials.Cancers (Basel). 2024 Jan 16;16(2):382. doi: 10.3390/cancers16020382. Cancers (Basel). 2024. PMID: 38254871 Free PMC article. Review.
-
Confirmation of Recurrent Lung Cancer Following Resection Using Liquid Biopsy, a Proof-of-Concept Real-World Study.Curr Oncol. 2024 Jul 17;31(7):4052-4062. doi: 10.3390/curroncol31070302. Curr Oncol. 2024. PMID: 39057174 Free PMC article.
-
Fulminant myocarditis during postoperative adjuvant chemotherapy for lung cancer with atezolizumab: a case report.J Med Case Rep. 2024 Mar 16;18(1):162. doi: 10.1186/s13256-024-04447-w. J Med Case Rep. 2024. PMID: 38491548 Free PMC article.
-
A comprehensive review of immune checkpoint inhibitor-related diabetes mellitus: incidence, clinical features, management, and prognosis.Front Immunol. 2024 Nov 4;15:1448728. doi: 10.3389/fimmu.2024.1448728. eCollection 2024. Front Immunol. 2024. PMID: 39559363 Free PMC article. Review.
-
Prognostic Value of Tertiary Lymphoid Structures in Stage I Nonsmall Cell Lung Cancer: Does Location Matter?Clin Med Insights Oncol. 2025 Apr 11;19:11795549251325061. doi: 10.1177/11795549251325061. eCollection 2025. Clin Med Insights Oncol. 2025. PMID: 40291840 Free PMC article.
References
-
- Remon J, Soria JC, Peters S, ESMO Guideline Committee . Early and locally advanced non‐small‐cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637‐1642. doi:10.1016/j.annonc.2021.08.1994 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

