The circadian system in cystic fibrosis mice is regulated by histone deacetylase 6
- PMID: 36062879
- PMCID: PMC9555305
- DOI: 10.1152/ajpcell.00248.2022
The circadian system in cystic fibrosis mice is regulated by histone deacetylase 6
Abstract
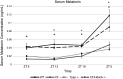
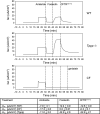
Disordered sleep experienced by people with cystic fibrosis (CF) suggest a possible disruption in circadian regulation being associated with the loss of cystic fibrosis transmembrane conductance regulator (Cftr) function. To test this hypothesis, circadian regulation was assessed in an F508del/F508del CF mouse model. CF mice exhibited significant alterations in both timing of locomotor activity and in mean activity per hour in both light-dark (LD) and dark-dark (DD) photoperiods compared with wild-type (WT) controls. It was also noted that in DD periodicity increased in CF mice, whereas shortening in WT mice as is expected. CF mice also exhibited altered timing of circadian gene expression and a reduction of melatonin production at all time points. Mechanistically, the role of microtubules in regulating these outcomes was explored. Mice lacking expression of tubulin polymerization promoting protein (Tppp) effectively mimicked CF mouse phenotypes with each measured outcome. Depleting expression of the microtubule regulatory protein histone deacetylase 6 (Hdac6) from CF mice (CF/Hdac6) resulted in the reversal of each phenotype to WT profiles. These data demonstrate an innate disruption of circadian regulation in CF mice and identify a novel microtubule-related mechanism leading to this disruption that can be targeted for therapeutic intervention.
Keywords: HDAC6; circadian; cystic fibrosis; melatonin; microtubule.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

