Issues with Tissues: Trends in Tissue-Engineered Products in Clinical Trials in the European Union
- PMID: 36062927
- PMCID: PMC9940800
- DOI: 10.1089/ten.TEB.2022.0094
Issues with Tissues: Trends in Tissue-Engineered Products in Clinical Trials in the European Union
Abstract
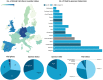
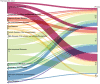
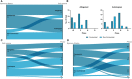
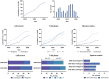
Tissue-engineered products (TEPs) consist of engineered cells or tissues produced to regenerate, repair, or replace a dysfunctional, diseased, or absent human tissue. TEPs make up <5% of all advanced therapeutic medicinal products (ATMPs) in clinical trials and received 5.1% of ATMP-designated funding in trials in the European Union (EU) in 2019, highlighting the relatively low proportion of TEPs being developed. The realization of TEPs being marketed has yet to be fulfilled, with few products being approved. Since 2009, 90 TEP-based clinical trials have been undertaken in the EU. Of these 90, 25 were Phase I/II trials, 35 were Phase II, 28 were Phase III, and two were Phase IV trials. This review provides an overview of TEPs in development, identifying musculoskeletal, cardiovascular, and skin/connective tissue disorders as the main therapeutic areas of interest. Commercial sponsors have funded most trials, and a significantly higher proportion of late-phase trials. Furthermore, this study has identified a shift toward the use of allogeneic cells in TEPs and increased activity in the proportion of early phase trials listed. This indicates a renewed interest in TEP development as sponsors adapt to the new regulation, with prospects of more TEP market authorization applications in the future. Impact Statement Tissue-engineered products (TEPs) consist of engineered cells or tissues produced to regenerate, repair, or replace a dysfunctional, diseased, or absent human tissue. This article evaluates the regulatory landscape of TEPs and identifies the trends in clinical trial activity in the European Union (EU) since the introduction of Regulation (EC) No 1394/2007. This article identifies trends in TEP development, highlighting the most active member states, commercial involvement, a shift toward the use of allogeneic cells and a renewed interest in TEP development in recent years.
Keywords: European Union; advanced therapeutic medicinal products; clinical trial; tissue-engineered product.
Conflict of interest statement
Z.B., G.R., and M.K.B. are employed by PharmaLex GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures

References
-
- The European Parliament and the Council of the European Union. Regulation (EC) no 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) no 726/2004. Off J Eur Union 2007;L324/121:1–17.
-
- Warreth S, Harris E. The regulatory landscape for ATMPs in the EU and US: A comparison. Lev 3 2020;15(2):5; doi: 10.21427/pk3v-g445 - DOI
-
- CAT Monthly Report of Application Procedure, Guidelines and Related Documents and Advance Therapies. May 2021 Meeting; 2021. Available from: www.ema.europa.eu/contact [Last accessed: July 30, 2021].
-
- Committee for Medicinal Products for Human Use. European Public Assessment Report for MACI. Procedure No. EMEA/H/C/002522/0000. London; 2013. Available from: https://www.ema.europa.eu/en/documents/assessment-report/maci-epar-publi... [Last accessed: June 1, 2021].
-
- Committee for Medicinal Products for Human Use. European Public Assessment Report for Chondrocelect. Procedure No. EMEA/H/C/000878. London; 2009. Available from: https://www.ema.europa.eu/en/documents/assessment-report/chondrocelect-e... [Last accessed: June 1, 2021].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
